Bioanalytics - Biomolecular
(M0930-06-36) 1-Tiered Approach for Anti-drug Antibody Testing in Clinical Study
Monday, October 21, 2024
9:30 AM - 10:30 AM MT
- JW
Jessica (Zheng) Wang, Ph.D.
Sr. Scientist
Regeneron Pharmaceuticals
Cresskill, New Jersey, United States - JW
Jessica (Zheng) Wang, Ph.D.
Sr. Scientist
Regeneron Pharmaceuticals
Cresskill, New Jersey, United States
Presenting Author(s)
Main Author(s)
Purpose: Therapeutic proteins have the potential to elicit anti-drug antibodies (ADA). Traditionally, a 3-tiered testing approach with rigorous cut points (CP) has been widely adopted to identify the presence (screening), drug specificity (confirmation), and magnitude (titer) of anti-drug antibody responses (FDA 2014, Mire-Sluis 2004, Shanker 2008, FDA EMA). However, the necessity of this 3-tiered approach has been challenged, as different tiers of ADA assessment have been shown to be highly correlated (Kubiak 2013, Starcevic Manning 2017,2022). In this study, we re-analyzed ADA data from a completed clinical study to evaluate if a simplified 1-tiered approach could generate comparable results.
Methods: To evaluate whether the confirmation and titer steps are providing additional insights in immunogenicity assessment, we conducted a statistical correlation analysis with results obtained from screening, confirmation, and titer assays. Subsequently, we reprocessed the screening signal-to-noise (S/N) data from the clinical study using a single CP (1-tiered CP). The CP was determined from the non-parametric method with 1% false positive rate (FPR). A sample with screening S/N ratio above the defined 1-tiered CP was reported as positive, otherwise the sample was ADA negative. The ADA category of each subject (negative, pre-existing, treatment emergent, etc.) was also reassessed based on the 1-tiered ADA results. Finally, we compared the pharmacokinetic (PK) profiles of subjects that were categorized differently with the 3-tiered approach versus the 1-tiered approach.
Results: A strong correlation was observed between the signal-to-noise (S/N) in screening assay and the inhibition ratio (fold signal reduction) in confirmation assay (Figure 1). Furthermore, the S/N in screening was strongly correlated to the titer level (Figure 1). To evaluate whether the 1-tiered approach would generate comparable results to the 3-tiered approach at both individual sample and subject levels, we reprocessed the screening S/N ratio data from the clinical study using the 1-tiered CP. Of these individual samples from subjects on active treatment, most samples (94.8%) had no change of status (Figure 2), and similarly a large majority of subjects (92.0%) had no change in ADA category using the 1-tiered approach (Figure 2). Analysis of the pharmacokinetic profiles revealed that the 1-tiered approach won’t impact assessment of ADA’s effect on drug exposure (Figure 3).
Conclusion: In this case study, we explored the approach of replacing the 3-tiered immunogenicity assessment approach with a 1-tiered approach. Correlation analysis suggested that the 3 tiers of assessment (screening, confirmation, and titer) provide very similar information. Furthermore, when we reprocessed the data, the 1-tiered approach aligned with the traditional 3-tiered approach in incidence of ADA at both the sample level and categorization at the subject level. This suggests 1-tiered approach could be a feasible alternative. In summary, the data indicate that the vast majority of patients (92%) had no change in ADA category when using the 1-tiered approach. Overall, our observations suggest the 1-tiered approach is a viable and efficient method for immunogenicity assessment in clinical studies.
References: 1. Food and Drug Administration. Immunogenicity assessment for therapeutic protein products. 2014.
2. Mire-Sluis, A. R., Y. C. Barrett, et al. (2004). "Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products." J Immunol Methods 289(1-2): 1-16.
3. Shankar, G., V. Devanarayan, et al. (2008). "Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products." J Pharm Biomed Anal 48(5): 1267-1281.
4. European Medicines Agency. Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use. 2012.
5. Kubiak RJ, Zhang L, Zhang J, et al. Correlation of screening and confirmatory results in tiered immunogenicity testing by solution-phase bridging assays. J Pharm Biomed Anal. 2013;74:235-245.
6. Starcevic Manning M, Kroenke MA, Lee SA, et al. Assay signal as an alternative to titer for assessment of magnitude of an antidrug antibody response. Bioanalysis. 2017;9(23):1849-1858.
7. Starcevic Manning M, Hassanein M, Partridge MA, et al. Comparison of Titer and Signal to Noise (S/N) for Determination of Anti-drug Antibody Magnitude Using Clinical Data from an Industry Consortium. AAPS J. 2022;24(4):81.
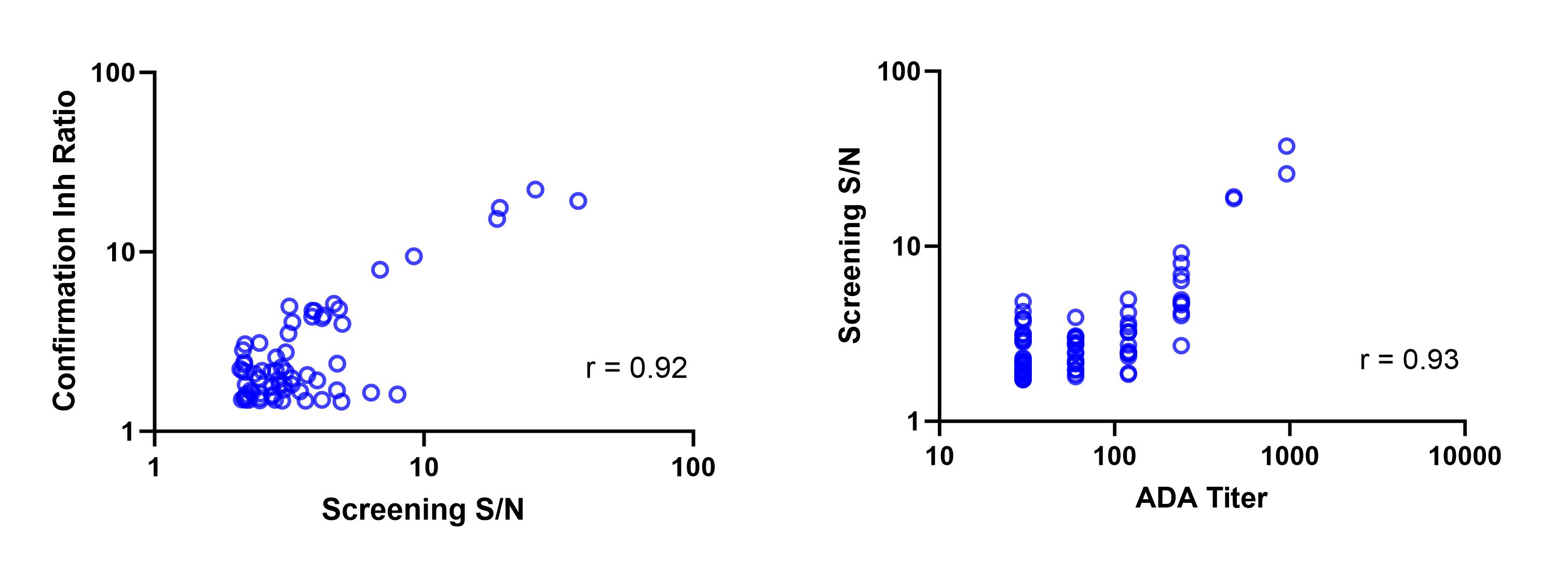
Figure 1.
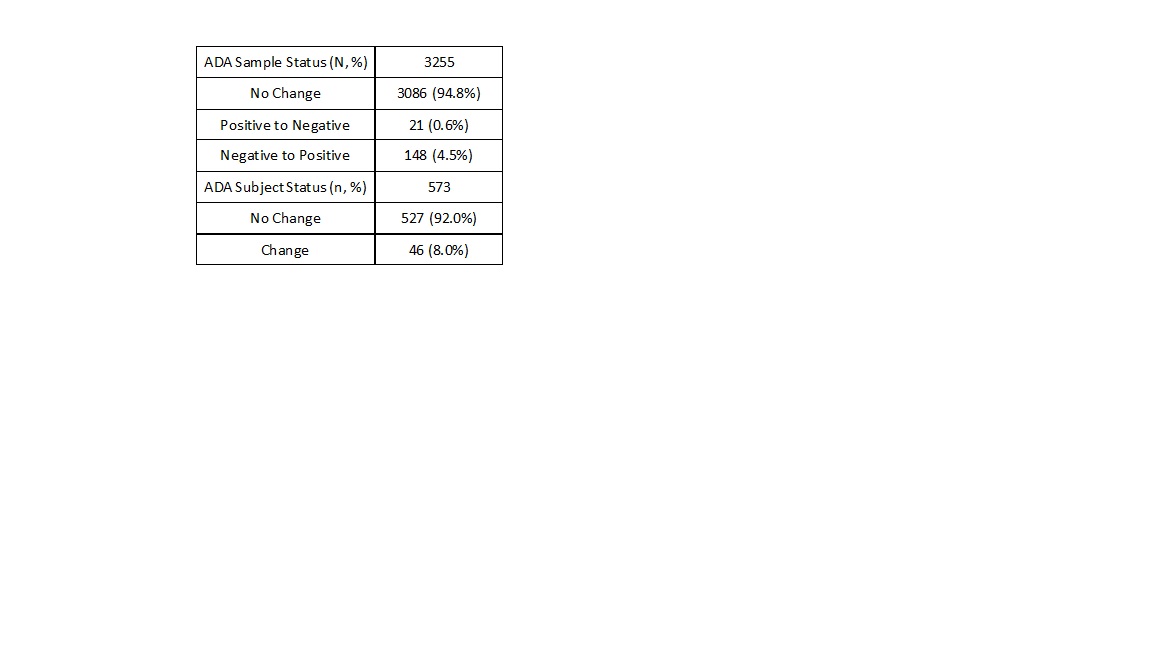
Figure 2.
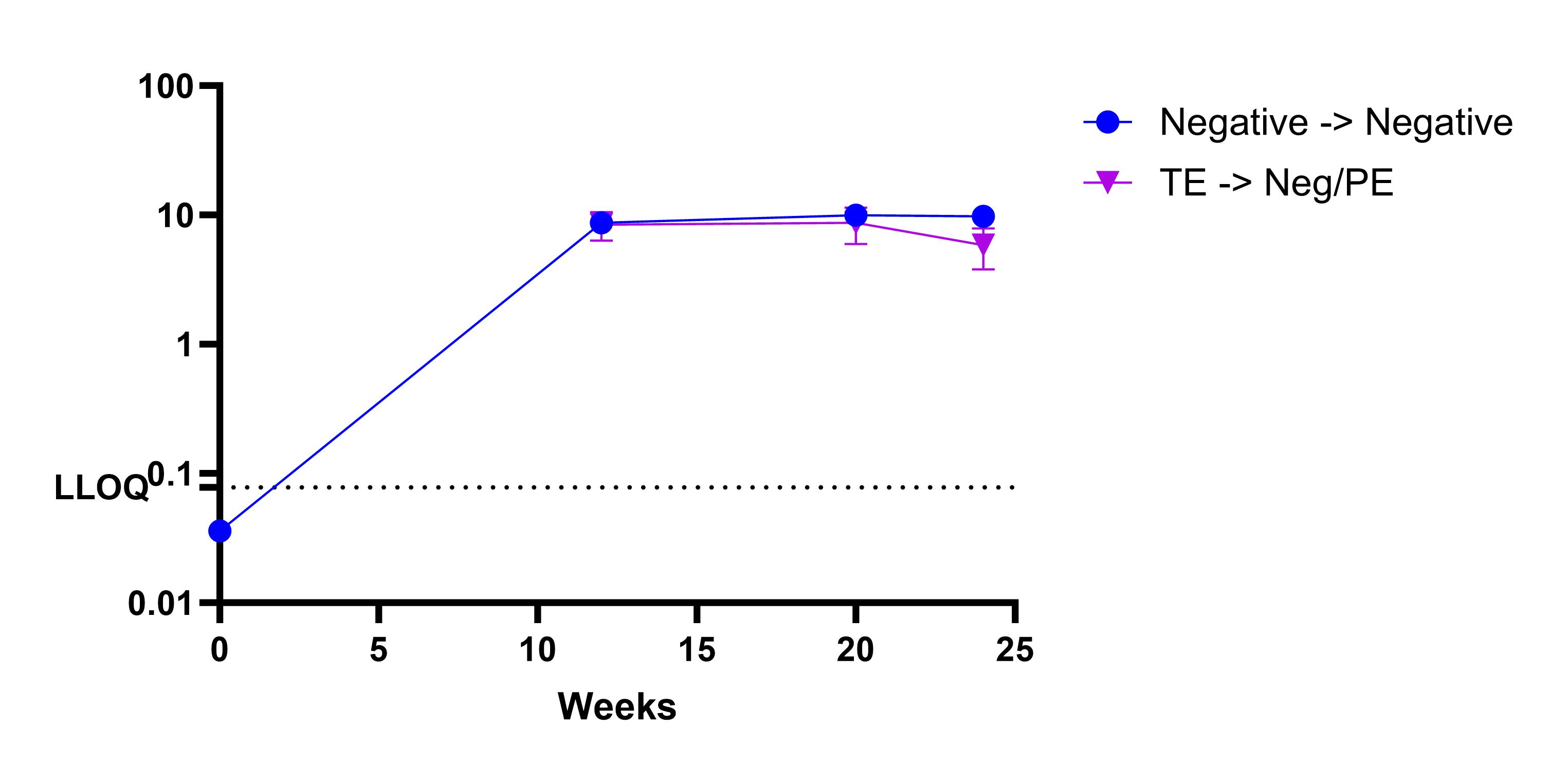
Figure 3.
Methods: To evaluate whether the confirmation and titer steps are providing additional insights in immunogenicity assessment, we conducted a statistical correlation analysis with results obtained from screening, confirmation, and titer assays. Subsequently, we reprocessed the screening signal-to-noise (S/N) data from the clinical study using a single CP (1-tiered CP). The CP was determined from the non-parametric method with 1% false positive rate (FPR). A sample with screening S/N ratio above the defined 1-tiered CP was reported as positive, otherwise the sample was ADA negative. The ADA category of each subject (negative, pre-existing, treatment emergent, etc.) was also reassessed based on the 1-tiered ADA results. Finally, we compared the pharmacokinetic (PK) profiles of subjects that were categorized differently with the 3-tiered approach versus the 1-tiered approach.
Results: A strong correlation was observed between the signal-to-noise (S/N) in screening assay and the inhibition ratio (fold signal reduction) in confirmation assay (Figure 1). Furthermore, the S/N in screening was strongly correlated to the titer level (Figure 1). To evaluate whether the 1-tiered approach would generate comparable results to the 3-tiered approach at both individual sample and subject levels, we reprocessed the screening S/N ratio data from the clinical study using the 1-tiered CP. Of these individual samples from subjects on active treatment, most samples (94.8%) had no change of status (Figure 2), and similarly a large majority of subjects (92.0%) had no change in ADA category using the 1-tiered approach (Figure 2). Analysis of the pharmacokinetic profiles revealed that the 1-tiered approach won’t impact assessment of ADA’s effect on drug exposure (Figure 3).
Conclusion: In this case study, we explored the approach of replacing the 3-tiered immunogenicity assessment approach with a 1-tiered approach. Correlation analysis suggested that the 3 tiers of assessment (screening, confirmation, and titer) provide very similar information. Furthermore, when we reprocessed the data, the 1-tiered approach aligned with the traditional 3-tiered approach in incidence of ADA at both the sample level and categorization at the subject level. This suggests 1-tiered approach could be a feasible alternative. In summary, the data indicate that the vast majority of patients (92%) had no change in ADA category when using the 1-tiered approach. Overall, our observations suggest the 1-tiered approach is a viable and efficient method for immunogenicity assessment in clinical studies.
References: 1. Food and Drug Administration. Immunogenicity assessment for therapeutic protein products. 2014.
2. Mire-Sluis, A. R., Y. C. Barrett, et al. (2004). "Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products." J Immunol Methods 289(1-2): 1-16.
3. Shankar, G., V. Devanarayan, et al. (2008). "Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products." J Pharm Biomed Anal 48(5): 1267-1281.
4. European Medicines Agency. Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use. 2012.
5. Kubiak RJ, Zhang L, Zhang J, et al. Correlation of screening and confirmatory results in tiered immunogenicity testing by solution-phase bridging assays. J Pharm Biomed Anal. 2013;74:235-245.
6. Starcevic Manning M, Kroenke MA, Lee SA, et al. Assay signal as an alternative to titer for assessment of magnitude of an antidrug antibody response. Bioanalysis. 2017;9(23):1849-1858.
7. Starcevic Manning M, Hassanein M, Partridge MA, et al. Comparison of Titer and Signal to Noise (S/N) for Determination of Anti-drug Antibody Magnitude Using Clinical Data from an Industry Consortium. AAPS J. 2022;24(4):81.

Figure 1.

Figure 2.

Figure 3.
