Formulation and Delivery - Chemical
(T1230-05-30) Controlled Release Mannose and RVG-9R Functionalized PLGA Nanoparticles for Targeting the Brain Viral Reservoir for the Management of HIV Associated Neurocognitive Disorder
Tuesday, October 22, 2024
12:30 PM - 1:30 PM MT
- JS
Jagdish Singh, Ph.D.
Department Chair
North Dakota State University
Fargo, North Dakota, United States - RT
Riddhi J. Trivedi, BS
Graduate Student
North Dakota State University
Fargo, North Dakota, United States
Presenting Author(s)
Main Author(s)
Purpose: The blood-brain barrier (BBB) poses an immense challenge in targeted drug delivery to the brain. The human immunodeficiency virus (HIV) is known to be neurotropic, allowing it to breach the BBB and resulting in various neurocognitive dysfunctions collectively referred to as HIV associated neurocognitive disorder (HAND). The inability of anti-retroviral drugs to effectively penetrate the BBB and maintain therapeutic concentrations over time in the brain leads to the formation of a viral reservoir in the brain. To tackle this issue, there is a need for a targeted drug delivery system capable of efficiently delivering anti-retroviral drugs to the brain parenchyma while ensuring controlled release. In this study, we have developed functionalized poly(lactic-co-glycolic acid) nanoparticles loaded with the anti-retroviral drugs Emtricitabine and Bictegravir. As the management of HIV often requires lifelong therapy involving daily drug intake, the utilization of controlled-release nanoparticles offers the dual benefit of targeting the brain's viral reservoir while also minimizing dosing frequency. The nanoparticles were functionalized with mannose to target the GLUT-1 transporters present on the blood-brain barrier; and cell-penetrating peptides Rabies virus glycoprotein-9R (RVG-9R) to enhance cellular penetration.
Methods: The in vitro evaluations encompassed studies on cytocompatibility, cellular uptake, transport across a blood-brain barrier model, and efficacy against microglia cells infected with Eco HIV. Furthermore, in vivo investigations involved assessing biodistribution and biocompatibility in c57b/6 mice. To accommodate high payloads of both hydrophilic (Emtricitabine) and hydrophobic (Bictegravir) drugs, the double emulsion technique was refined to prepare the nanoparticles. The resultant formulation underwent characterization for size, zeta potential, and polydispersity index (PDI) using dynamic light scattering (DLS). In vitro release and entrapment efficiency of the nanoparticles were determined using RP/HPLC-UV. Moreover, the functionalized nanoparticles were assessed for cytocompatibility and cellular uptake in bEnd.3 cells and astrocytes, while their hemolytic activity against RBCs was investigated. The transportability of the formulation was evaluated through an in vitro blood-brain barrier model. Additionally, the formulation's ability to reduce the viral reservoir in vitro was determined using Eco-HIV infected microglia cells, with viral load quantified using a p24 viral protein ELISA kit. C57BL/6 mice were administered intravenous injections of 40 mg/kg of Emtricitabine-equivalent PLGA nanoparticles or a free drug mixture. Animals were sacrificed at various time points (1h, 8h, 16h, 24h, 72h, 168h, and 240h) for organ collection. Furthermore, the biocompatibility of the nanoparticles was assessed through histological examination of tissue sections collected after 24 hours and 10 days of nanoparticle, free drug, and PBS treatment.
Results: The optimized nanoparticles, loaded with two drugs and functionalized with dual modifications, exhibited a consistent size distribution and spherical morphology as observed in TEM images. Their particle size measured at 246.7 ± 1.53nm, with a zeta potential of -6.15 ± 0.97mV, and a PDI of 0.235 ± 0.054. These nanoparticles demonstrated controlled release of both drugs, with 90.3 ± 10.7% of Emtricitabine and 85 ± 4.7% of Bictegravir released over a span of 10 days. Cell viability assays confirmed the formulations' non-toxicity to bEnd.3 cells and astrocytes. Furthermore, the nanoparticles exhibited minimal hemolysis, with less than 10% observed, indicating their safety for intravenous administration. Moreover, the modified nanoparticles showed significantly enhanced cellular uptake and transport across an in vitro blood-brain barrier (BBB) model compared to plain nanoparticles and free drugs, while maintaining the barrier's integrity, as evidenced by consistent TEER values before and after treatment. In vitro efficacy studies revealed that the dual-functionalized (RVGMan) nanoparticles significantly reduced viral protein p24 levels compared to both the free drugs and plain nanoparticles. In vivo investigations in C57BL/6 mice demonstrated that drug concentrations delivered via nanoparticles peaked at 16 hours post-treatment and remained elevated for up to 10 days, whereas free drugs were eliminated from the brain after 24 hours. This sustained release profile offers the advantage of continuous therapeutic efficacy, potentially reducing the need for frequent dosing.
Conclusion: The double emulsion PLGA nanoparticles exhibited a uniform size distribution. The incorporation of mannose and Rabies virus glycoprotein-9R (RVGManNP) led to enhanced cellular uptake, transport across an in vitro blood-brain barrier (BBB) model, and efficacy against Eco-HIV-infected cells. Moreover, these modified nanoparticles demonstrated favorable biocompatibility with red blood cells (RBCs), bEND.3 cells, and astrocytes. Furthermore, the dual-functionalized nanoparticles exhibited sustained drug release in the brain over a period of 10 days, suggesting the potential for reduced dosing frequency. These findings suggest that RVG and mannose-functionalized polymeric nanoparticles can effectively transport both drugs across the blood-brain barrier in a controlled-release manner, targeting the brain's viral reservoir and potentially addressing HIV-associated neurocognitive disorder.
Acknowledgements: This research was supported by the National Institutes of Health (NIH) Grants R01 AG051574, RF1 AG068034 and 3RF1AG068034-01A1S1.
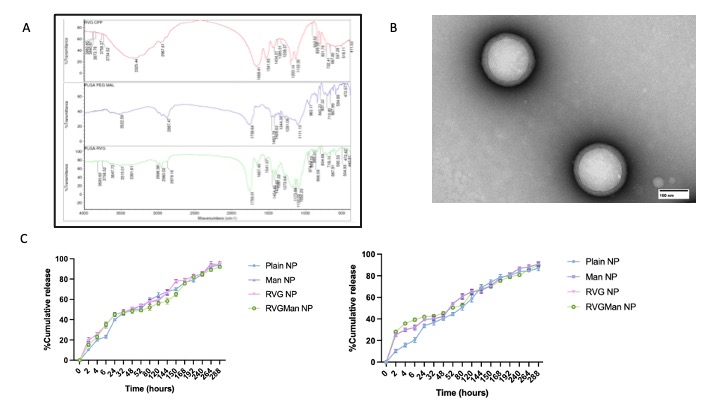
Figure 1: (A) FTIR Spectra of PLGA-PEG-Mal, PLGA-PEG-RVG, Rabies virus glycoprotein-9R (RVG) CPP. (B) Transmission electron microscopy (TEM) images of RVGMan Nanoparticles, which were negatively stained with 0.1% phosphotungstic acid aqueous solution (Scale 50 nm). (C) Percent cumulative release of Emtricitabine andBictegravir from plain, Man, RVG, and RVGMan nanoparticles. No statistically significant (p < 0.05) differences were seen between the groups. Data represented as mean ± S.D. (n=4).
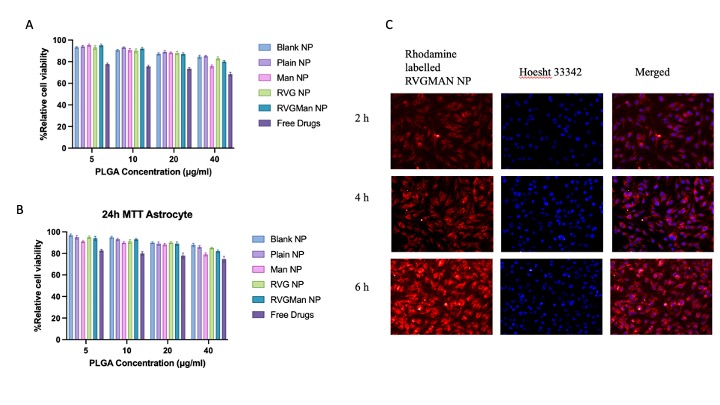
Figure 2: In-vitro cytocompatibility of different PLGA nanoparticles formulations and free drugs in (A) brain endothelial cells, (B) primary astrocytes. Cellular viability of free drug and PLGA at different concentration was evaluated upon exposure for 24 h Data represented as mean ± S.D. (n = 6). (C) Fluorescence microscopic images (20X magnification) showing cellular uptake of rhodamine labeled RVGMan NP in bEnd.3 cells after 2 h, 4 h and 6 h of incubation with treatment. The nuclei of the cells were stained with Hoechst 33342. The images show the overlap of rhodamine (red) and nuclei of the cells (blue).
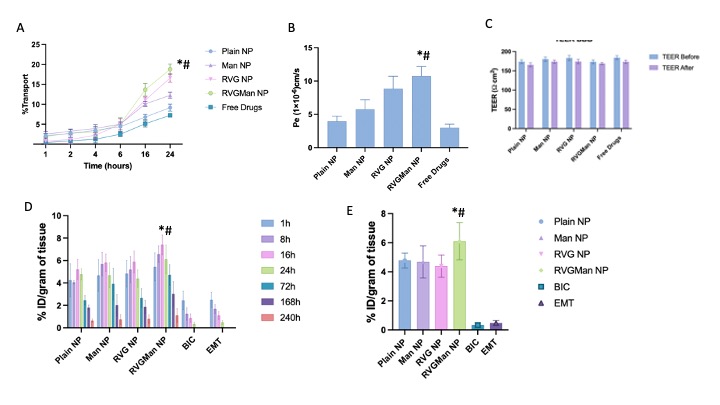
Figure 3: A) Transport through BBB model over a period of 24 h. (B) Endothelial cell permeability coefficient (Pe, expressed in 1x10-6 cm/s). (C) In-vitro viral suppression after treatment with different PLGA nanoparticles and free drug. Data represented as mean ± S.D. (n = 4). (D) Biodistribution of different nanoparticle and free drugs after 1 h, 8h, 16h, 24 h, 72 h, 168 h, and 240 h of intravenous injection in C57BL/6 mice in Brain, (E) Biodistribution of different nanoparticle and free drugs in brain after 24h of intravenous injection in C57BL/6 mice in Brain. Data are expressed as mean ± SD of injected dose percentage (%ID) per gram of tissue. Statistically significant (p < 0.01) differences are shown as (*) with the free drug treatment group and (#) with Plain nanoparticles.
Methods: The in vitro evaluations encompassed studies on cytocompatibility, cellular uptake, transport across a blood-brain barrier model, and efficacy against microglia cells infected with Eco HIV. Furthermore, in vivo investigations involved assessing biodistribution and biocompatibility in c57b/6 mice. To accommodate high payloads of both hydrophilic (Emtricitabine) and hydrophobic (Bictegravir) drugs, the double emulsion technique was refined to prepare the nanoparticles. The resultant formulation underwent characterization for size, zeta potential, and polydispersity index (PDI) using dynamic light scattering (DLS). In vitro release and entrapment efficiency of the nanoparticles were determined using RP/HPLC-UV. Moreover, the functionalized nanoparticles were assessed for cytocompatibility and cellular uptake in bEnd.3 cells and astrocytes, while their hemolytic activity against RBCs was investigated. The transportability of the formulation was evaluated through an in vitro blood-brain barrier model. Additionally, the formulation's ability to reduce the viral reservoir in vitro was determined using Eco-HIV infected microglia cells, with viral load quantified using a p24 viral protein ELISA kit. C57BL/6 mice were administered intravenous injections of 40 mg/kg of Emtricitabine-equivalent PLGA nanoparticles or a free drug mixture. Animals were sacrificed at various time points (1h, 8h, 16h, 24h, 72h, 168h, and 240h) for organ collection. Furthermore, the biocompatibility of the nanoparticles was assessed through histological examination of tissue sections collected after 24 hours and 10 days of nanoparticle, free drug, and PBS treatment.
Results: The optimized nanoparticles, loaded with two drugs and functionalized with dual modifications, exhibited a consistent size distribution and spherical morphology as observed in TEM images. Their particle size measured at 246.7 ± 1.53nm, with a zeta potential of -6.15 ± 0.97mV, and a PDI of 0.235 ± 0.054. These nanoparticles demonstrated controlled release of both drugs, with 90.3 ± 10.7% of Emtricitabine and 85 ± 4.7% of Bictegravir released over a span of 10 days. Cell viability assays confirmed the formulations' non-toxicity to bEnd.3 cells and astrocytes. Furthermore, the nanoparticles exhibited minimal hemolysis, with less than 10% observed, indicating their safety for intravenous administration. Moreover, the modified nanoparticles showed significantly enhanced cellular uptake and transport across an in vitro blood-brain barrier (BBB) model compared to plain nanoparticles and free drugs, while maintaining the barrier's integrity, as evidenced by consistent TEER values before and after treatment. In vitro efficacy studies revealed that the dual-functionalized (RVGMan) nanoparticles significantly reduced viral protein p24 levels compared to both the free drugs and plain nanoparticles. In vivo investigations in C57BL/6 mice demonstrated that drug concentrations delivered via nanoparticles peaked at 16 hours post-treatment and remained elevated for up to 10 days, whereas free drugs were eliminated from the brain after 24 hours. This sustained release profile offers the advantage of continuous therapeutic efficacy, potentially reducing the need for frequent dosing.
Conclusion: The double emulsion PLGA nanoparticles exhibited a uniform size distribution. The incorporation of mannose and Rabies virus glycoprotein-9R (RVGManNP) led to enhanced cellular uptake, transport across an in vitro blood-brain barrier (BBB) model, and efficacy against Eco-HIV-infected cells. Moreover, these modified nanoparticles demonstrated favorable biocompatibility with red blood cells (RBCs), bEND.3 cells, and astrocytes. Furthermore, the dual-functionalized nanoparticles exhibited sustained drug release in the brain over a period of 10 days, suggesting the potential for reduced dosing frequency. These findings suggest that RVG and mannose-functionalized polymeric nanoparticles can effectively transport both drugs across the blood-brain barrier in a controlled-release manner, targeting the brain's viral reservoir and potentially addressing HIV-associated neurocognitive disorder.
Acknowledgements: This research was supported by the National Institutes of Health (NIH) Grants R01 AG051574, RF1 AG068034 and 3RF1AG068034-01A1S1.

Figure 1: (A) FTIR Spectra of PLGA-PEG-Mal, PLGA-PEG-RVG, Rabies virus glycoprotein-9R (RVG) CPP. (B) Transmission electron microscopy (TEM) images of RVGMan Nanoparticles, which were negatively stained with 0.1% phosphotungstic acid aqueous solution (Scale 50 nm). (C) Percent cumulative release of Emtricitabine andBictegravir from plain, Man, RVG, and RVGMan nanoparticles. No statistically significant (p < 0.05) differences were seen between the groups. Data represented as mean ± S.D. (n=4).

Figure 2: In-vitro cytocompatibility of different PLGA nanoparticles formulations and free drugs in (A) brain endothelial cells, (B) primary astrocytes. Cellular viability of free drug and PLGA at different concentration was evaluated upon exposure for 24 h Data represented as mean ± S.D. (n = 6). (C) Fluorescence microscopic images (20X magnification) showing cellular uptake of rhodamine labeled RVGMan NP in bEnd.3 cells after 2 h, 4 h and 6 h of incubation with treatment. The nuclei of the cells were stained with Hoechst 33342. The images show the overlap of rhodamine (red) and nuclei of the cells (blue).

Figure 3: A) Transport through BBB model over a period of 24 h. (B) Endothelial cell permeability coefficient (Pe, expressed in 1x10-6 cm/s). (C) In-vitro viral suppression after treatment with different PLGA nanoparticles and free drug. Data represented as mean ± S.D. (n = 4). (D) Biodistribution of different nanoparticle and free drugs after 1 h, 8h, 16h, 24 h, 72 h, 168 h, and 240 h of intravenous injection in C57BL/6 mice in Brain, (E) Biodistribution of different nanoparticle and free drugs in brain after 24h of intravenous injection in C57BL/6 mice in Brain. Data are expressed as mean ± SD of injected dose percentage (%ID) per gram of tissue. Statistically significant (p < 0.01) differences are shown as (*) with the free drug treatment group and (#) with Plain nanoparticles.
