Bioanalytics - Biomolecular
(T1230-06-34) PEGylated Nanoparticles Conjugated with LHRH Receptor Binding Peptide for Treatment of Lung Cancer
Tuesday, October 22, 2024
12:30 PM - 1:30 PM MT
- SN
Sterling Neill, PhD
student
Mercer University
dallas, Georgia, United States - MC
Mahavir Chougule, Ph.D. (he/him/his)
Associate Professor
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Purpose: The aim was to synthesize GSH-responsive poly (amino) ether polymer which was used to develop, characterize, and analyze the MS-loaded modified poly (amino ether) based polymeric pegylated nanoparticles conjugated with LHRH-R binding peptide product (MS-MP-PG-LR) nanoparticles (NPs). The MS-MP-PG-LR-NPs delivery of MS for mTOR gene silencing, decreased NSCLC cell viability, which downregulated mTOR siRNA gene expression, thereby inhibiting NSCLC cell growth.
Methods: The mTOR siRNA encapsulated PEGylated and targeted nanoparticles (PEG-LHRH NPs) were prepared by a modified nanoprecipitation method14 The surface-modified NPs with PEG and LHRH-R binding peptide (MS-MP-PG-LR) were synthesized by a two-step reaction. To achieve a target PEG% of 20-25% on the surface of MS-MP-NPs, two times excess of PEG-AMAS (4 mg) reacted with 10 mg of MS-MP-NPs (equivalent to 10 mg of mPAE polymers). The nanoprecipitation method was used to formulate LHRH-R targeted NPs in two steps. For the determination of particle size distribution (PSD), polydispersity index (PDI) and the zeta potential(ZP) of the mTOR siRNA-mPAE-PEG and mTOR siRNA-mPAE-PEG-LHRH 20 s, they were prepared at weight ratios of 45:1 (mPAE: siRNA) and were analyzed using NICOMP 380ZLS (Port Richey, FL, USA) in the intensity mode14]. The cytotoxicity of the formulations was evaluated using a modified Sulforhodamine B (SRB) assay. For the evaluation of the mTOR siRNA effects on cell proliferation, mTOR siRNA-mPAE-PEG-LHRH-R binding peptide nanoparticles (MS-MP-PG-LR20 NPs) were incubated with A549 and H460 cell lines.
Results: PEGylated MP-NPs exhibited decreased cytotoxicity compared to non-PEGylated MP-NPs in A549 and H460 cells (**p < 0.01). The cell viability (%) and expression of the mTOR proteins were significantly decreased after treatment with MS-MP-PG-LR NPs in A549 and H460 cells (**p < 0.01). The LHRH-R was found to be significantly (***p < 0.001) overexpressed in A549 and H460 cells compared to the ovarian cancer cell line SKOV-3. The densitometry analysis shows that there is a nearly 4-fold and 4.5-fold times overexpression of LHRH-R in A549 and H460 cells compared to the SKOV-3 cells. The cell viability (%) of non-targeted MP-NP formulation in three different concentrations of 15 µg/ml, 30 µg/ml and 60 µg/ml (93.33 ± 7.6%, 83.7 ± 8.2%, and 65.4 ± 10.4%) and (94.33 ± 6.60%, 82.33 ± 13.60%, and 60.81 ± 8.59%) in A549 and H460 cells, respectively. Western blot analysis was performed to evaluate the mTOR gene silencing efficiency of the non-targeted MP-NP, MP-PG-NP(PEGylated) and MP-PG-LR-NP (PEGylated-LHRH-R targeted) at different concentrations (15 µg/ml, 30 µg/ml, and 60 µg/ml) in A549 and H460 cells, after 48 h treatment. We investigated the mTOR silencing efficiency of MS-MP-PG-LR20 nanoparticles (using 50 nM and 100 nM siRNA) in human lung cancer cell lines A549 and H460 cells by applying nanoparticles at the fixed weight ratio of 45:1 (mPAE: mTOR siRNA). The scrambled siRNA (SS)-based mPAE nanoparticles were also prepared as the control. The negative control (SS-MP-PG-LR20 NPs) did not show gene silencing effects in the A549 and H460 cell lines at 50 nM and 100 nM concentrations. The effect of mTOR siRNA encapsulated targeted nanoparticles on apoptosis in A549 and H460 cells, was evaluated by using SRB assay. Cell viability (%) of mTOR siRNA-mPAE-PEG-LHRH-R binding peptide nanoparticles (MS-MP-PG-LR20 NPs) were significantly lower than the negative scrambled siRNA (SS) control-based nanoparticles (SS-MP-PG-LR20 NPs) and placebo nanoparticles (MP-PG-LR20 NPs) in both A549 and H460 cells (**p < 0.01).
Conclusion: MS-MP-PG-LR efficiently silenced the mTOR gene in A549 and H460 cells. These findings are significant as they offer a novel approach to combating NSCLC, a prevalent and treatment-resistant form of lung cancer, therefore providing promise for improved patient outcomes and survival rates. Not only does this study provide evidence for the importance for developing siRNA delivery systems that improve upon current tumor penetration abilities for patients suffering from NSCLC, but also suggests the applicability of using siRNA delivery systems to combat other cancers.
References: K. C. Thandra, A. Barsouk, K. Saginala, J. S. Aluru, A. Barsouk, Contemp Oncol (Pozn) 2021, 25, 45-52.
[2] A. K. Kotha, R. Kashikar, P. Famta, S. Shah, S. Srivastava, M. B. Chougule, in Nanomaterials for Cancer Detection Using Imaging Techniques and Their Clinical Applications, Springer, 2022, pp. 225-259.
[3] R. L. Siegel, K. D. Miller, A. Jemal, CA Cancer J Clin 2020, 70, 7-30.
[4] R. Chen, R. Manochakian, L. James, A. G. Azzouqa, H. Shi, Y. Zhang, Y. Zhao, K. Zhou, Y. Lou, J Hematol Oncol 2020, 13, 58.
[5] V. Muthu, B. Mylliemngap, K. T. Prasad, D. Behera, N. Singh, Lung India 2019, 36, 32-37.
[6] S. Tan, D. Li, X. Zhu, Biomed Pharmacother 2020, 124, 109821.
[7] A. C. Tan, Thorac Cancer 2020, 11, 511-518.
[8] H. Hua, Q. Kong, H. Zhang, J. Wang, T. Luo, Y. Jiang, J Hematol Oncol 2019, 12, 71.
[9] N. Pallet, C. Legendre, Expert Opin Drug Saf 2013, 12, 177-186.
[10] aN. S. Gandhi, R. K. Tekade, M. B. Chougule, J Control Release 2014, 194, 238-256; bY. Hattori, M. Nakamura, N. Takeuchi, K. Tamaki, S. Shimizu, Y. Yoshiike, M. Taguchi, H. Ohno, K. I. Ozaki, H. Onishi, J Drug Target 2019, 27, 217-227.
[11] Y. S. Lee, S. W. Kim, J Control Release 2014, 190, 424-439.
[12] aJ. Ramos, T. Potta, O. Scheideler, K. Rege, ACS Appl Mater Interfaces 2014, 6, 14861-14873; bT. Potta, Z. Zhen, T. S. Grandhi, M. D. Christensen, J. Ramos, C. M. Breneman, K. Rege, Biomaterials 2014, 35, 1977-1988; cS. Goklany, P. Lu, S. Godeshala, A. Hall, E. Garrett-Mayer, C. Voelkel-Johnson, K. Rege, J Mater Chem B 2019, 7, 7014-7025; dS. Godeshala, B. Miryala, S. Dutta, M. D. Christensen, P. Nandi, P. L. Chiu, K. Rege, J Mater Chem B 2020, 8, 8558-8572.
[13] S. Kommareddy, M. Amiji, Bioconjug Chem 2005, 16, 1423-1432.
[14] N. S. Gandhi, S. Godeshala, D. T. Koomoa-Lange, B. Miryala, K. Rege, M. B. Chougule, Pharm Res 2018, 35, 188.
[15] H. H. Gustafson, D. Holt-Casper, D. W. Grainger, H. Ghandehari, Nano Today 2015, 10, 487-510.
[16] L. Shi, J. Zhang, M. Zhao, S. Tang, X. Cheng, W. Zhang, W. Li, X. Liu, H. Peng, Q. Wang, Nanoscale 2021, 13, 10748-10764.
[17] J. S. Suk, Q. Xu, N. Kim, J. Hanes, L. M. Ensign, Adv Drug Deliv Rev 2016, 99, 28-51.
[18] S. Sundaram, R. Trivedi, C. Durairaj, R. Ramesh, B. K. Ambati, U. B. Kompella, Clin Cancer Res 2009, 15, 7299-7308.
[19] X. Li, O. Taratula, O. Taratula, C. Schumann, T. Minko, Mini Rev Med Chem 2017, 17, 258-267.
[20] T. Minko, M. L. Patil, M. Zhang, J. J. Khandare, M. Saad, P. Chandna, O. Taratula, Methods Mol Biol 2010, 624, 281-294.
[21] aS. S. Dharap, Y. Wang, P. Chandna, J. J. Khandare, B. Qiu, S. Gunaseelan, P. J. Sinko, S. Stein, A. Farmanfarmaian, T. Minko, Proc Natl Acad Sci U S A 2005, 102, 12962-12967; bS. S. Dharap, B. Qiu, G. C. Williams, P. Sinko, S. Stein, T. Minko, J Control Release 2003, 91, 61-73.
[22] H. Gosnell, L. M. Kasman, T. Potta, L. Vu, E. Garrett-Mayer, K. Rege, C. Voelkel-Johnson, Journal of Controlled Release 2014, 176, 35-43.
[23] V. Vichai, K. Kirtikara, Nat Protoc 2006, 1, 1112-1116.
[24] aK. Hara, H. Tsujimoto, C. C. Huang, Y. Kawashima, R. Ando, O. Kusuoka, K. Tamura, M. Tsutsumi, J Toxicol Pathol 2012, 25, 19-26; bC. W. Liu, W. J. Lin, Int J Nanomedicine 2012, 7, 4749-4767.
[25] Q. Xu, L. M. Ensign, N. J. Boylan, A. Schon, X. Gong, J. C. Yang, N. W. Lamb, S. Cai, T. Yu, E. Freire, J. Hanes, ACS Nano 2015, 9, 9217-9227.
[26] C. G. Doss, S. Debottam, C. Debajyoti, Protoplasma 2013, 250, 787-792.
[27] E. Carrasco-Esteban, J. A. Domínguez-Rullán, P. Barrionuevo-Castillo, L. Pelari-Mici, O. Leaman, S. Sastre-Gallego, F. López-Campos, J Clin Transl Res 2021, 7, 140-155.
[28] P. Foroozandeh, A. A. Aziz, Nanoscale Res Lett 2018, 13, 339.
[29] T. S. Hauck, A. A. Ghazani, W. C. Chan, Small 2008, 4, 153-159.
[30] Y. W. Huang, M. Cambre, H. J. Lee, Int J Mol Sci 2017, 18.
[31] Y. Dai, X. Zhang, Macromol Biosci 2019, 19, e1800445.
[32] A. Hall, U. Lächelt, J. Bartek, E. Wagner, S. M. Moghimi, Mol Ther 2017, 25, 1476-1490.
[33] J. S. Suk, Q. Xu, N. Kim, J. Hanes, L. M. Ensign, Advanced drug delivery reviews 2016, 99, 28-51.
[34] aH. Y. Xue, S. Liu, H. L. Wong, Nanomedicine (Lond) 2014, 9, 295-312; bR. Bholakant, H. Qian, J. Zhang, X. Huang, D. Huang, J. Feijen, Y. Zhong, W. Chen, Biomacromolecules 2020, 21, 2966-2982.
[35] C. Li, H. Liu, Y. Sun, H. Wang, F. Guo, S. Rao, J. Deng, Y. Zhang, Y. Miao, C. Guo, J. Meng, X. Chen, L. Li, D. Li, H. Xu, H. Wang, B. Li, C. Jiang, J Mol Cell Biol 2009, 1, 37-45.
[36] aN. Man, Y. Chen, F. Zheng, W. Zhou, L. P. Wen, Autophagy 2010, 6, 449-454; bJ. Zhao, Z. Li, M. Wang, Z. Zhang, H. Ma, J. Chang, D. Gao, S. Wang, Acta Biochimica et Biophysica Sinica 2013, 45, 979-981.
[37] S. F. Dowdy, Nat Biotechnol 2017, 35, 222-229.
[38] K. Koushik, N. Bandi, S. Sundaram, U. B. Kompella, Pharm Res 2004, 21, 1034-1046.
[39] A. Sharma, D. Shambhwani, S. Pandey, J. Singh, H. Lalhlenmawia, M. Kumarasamy, S. K. Singh, D. K. Chellappan, G. Gupta, P. Prasher, K. Dua, D. Kumar, ACS Omega 2023, 8, 10-41.
[40] N. V. Nukolova, H. S. Oberoi, Y. Zhao, V. P. Chekhonin, A. V. Kabanov, T. K. Bronich, Mol Pharm 2013, 10, 3913-3921.
[41] H. Yang, S. Y. Fung, S. Xu, D. P. Sutherland, T. R. Kollmann, M. Liu, S. E. Turvey, ACS Nano 2015, 9, 6774-6784.
[42] C. Leuschner, C. S. Kumar, W. Hansel, W. Soboyejo, J. Zhou, J. Hormes, Breast Cancer Research and Treatment 2006, 99, 163-176.
[43] aJ. D. Obayemi, A. A. Salifu, S. C. Eluu, V. O. Uzonwanne, S. M. Jusu, C. C. Nwazojie, C. E. Onyekanne, O. Ojelabi, L. Payne, C. M. Moore, J. A. King, W. O. Soboyejo, Sci Rep 2020, 10, 8212; bM. S. Kim, S. Ma, A. Chelariu-Raicu, C. Leuschner, H. W. Alila, S. Lee, R. L. Coleman, A. K. Sood, Mol Cancer Ther 2020, 19, 2396-2406.
Acknowledgements: Dr. Rege is affiliated with Synergyan, LLC. Dr. Chougule is affiliated with Adexp Biopharm Consulting, Litigation and Patent Experts LLC.
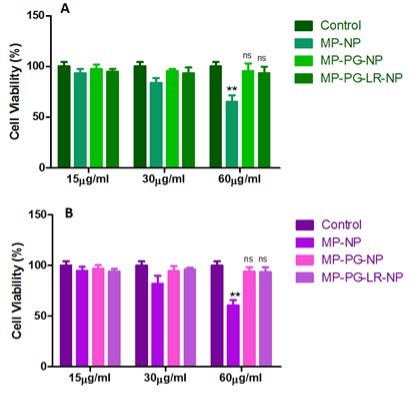
Figure 1. In vitro cell viability of non-PEGylated mPAE (MP-NP), PEGylated mPAE (MP-PG-NP) and PEGylated, targeted mPAE (MP-PG-LR-NP) nanoparticles (without siRNA loaded) treated A549 and H460 cells at various concentrations (15µg/ml, 30 µg/ml, and 60 µg/ml).
Methods: The mTOR siRNA encapsulated PEGylated and targeted nanoparticles (PEG-LHRH NPs) were prepared by a modified nanoprecipitation method14 The surface-modified NPs with PEG and LHRH-R binding peptide (MS-MP-PG-LR) were synthesized by a two-step reaction. To achieve a target PEG% of 20-25% on the surface of MS-MP-NPs, two times excess of PEG-AMAS (4 mg) reacted with 10 mg of MS-MP-NPs (equivalent to 10 mg of mPAE polymers). The nanoprecipitation method was used to formulate LHRH-R targeted NPs in two steps. For the determination of particle size distribution (PSD), polydispersity index (PDI) and the zeta potential(ZP) of the mTOR siRNA-mPAE-PEG and mTOR siRNA-mPAE-PEG-LHRH 20 s, they were prepared at weight ratios of 45:1 (mPAE: siRNA) and were analyzed using NICOMP 380ZLS (Port Richey, FL, USA) in the intensity mode14]. The cytotoxicity of the formulations was evaluated using a modified Sulforhodamine B (SRB) assay. For the evaluation of the mTOR siRNA effects on cell proliferation, mTOR siRNA-mPAE-PEG-LHRH-R binding peptide nanoparticles (MS-MP-PG-LR20 NPs) were incubated with A549 and H460 cell lines.
Results: PEGylated MP-NPs exhibited decreased cytotoxicity compared to non-PEGylated MP-NPs in A549 and H460 cells (**p < 0.01). The cell viability (%) and expression of the mTOR proteins were significantly decreased after treatment with MS-MP-PG-LR NPs in A549 and H460 cells (**p < 0.01). The LHRH-R was found to be significantly (***p < 0.001) overexpressed in A549 and H460 cells compared to the ovarian cancer cell line SKOV-3. The densitometry analysis shows that there is a nearly 4-fold and 4.5-fold times overexpression of LHRH-R in A549 and H460 cells compared to the SKOV-3 cells. The cell viability (%) of non-targeted MP-NP formulation in three different concentrations of 15 µg/ml, 30 µg/ml and 60 µg/ml (93.33 ± 7.6%, 83.7 ± 8.2%, and 65.4 ± 10.4%) and (94.33 ± 6.60%, 82.33 ± 13.60%, and 60.81 ± 8.59%) in A549 and H460 cells, respectively. Western blot analysis was performed to evaluate the mTOR gene silencing efficiency of the non-targeted MP-NP, MP-PG-NP(PEGylated) and MP-PG-LR-NP (PEGylated-LHRH-R targeted) at different concentrations (15 µg/ml, 30 µg/ml, and 60 µg/ml) in A549 and H460 cells, after 48 h treatment. We investigated the mTOR silencing efficiency of MS-MP-PG-LR20 nanoparticles (using 50 nM and 100 nM siRNA) in human lung cancer cell lines A549 and H460 cells by applying nanoparticles at the fixed weight ratio of 45:1 (mPAE: mTOR siRNA). The scrambled siRNA (SS)-based mPAE nanoparticles were also prepared as the control. The negative control (SS-MP-PG-LR20 NPs) did not show gene silencing effects in the A549 and H460 cell lines at 50 nM and 100 nM concentrations. The effect of mTOR siRNA encapsulated targeted nanoparticles on apoptosis in A549 and H460 cells, was evaluated by using SRB assay. Cell viability (%) of mTOR siRNA-mPAE-PEG-LHRH-R binding peptide nanoparticles (MS-MP-PG-LR20 NPs) were significantly lower than the negative scrambled siRNA (SS) control-based nanoparticles (SS-MP-PG-LR20 NPs) and placebo nanoparticles (MP-PG-LR20 NPs) in both A549 and H460 cells (**p < 0.01).
Conclusion: MS-MP-PG-LR efficiently silenced the mTOR gene in A549 and H460 cells. These findings are significant as they offer a novel approach to combating NSCLC, a prevalent and treatment-resistant form of lung cancer, therefore providing promise for improved patient outcomes and survival rates. Not only does this study provide evidence for the importance for developing siRNA delivery systems that improve upon current tumor penetration abilities for patients suffering from NSCLC, but also suggests the applicability of using siRNA delivery systems to combat other cancers.
References: K. C. Thandra, A. Barsouk, K. Saginala, J. S. Aluru, A. Barsouk, Contemp Oncol (Pozn) 2021, 25, 45-52.
[2] A. K. Kotha, R. Kashikar, P. Famta, S. Shah, S. Srivastava, M. B. Chougule, in Nanomaterials for Cancer Detection Using Imaging Techniques and Their Clinical Applications, Springer, 2022, pp. 225-259.
[3] R. L. Siegel, K. D. Miller, A. Jemal, CA Cancer J Clin 2020, 70, 7-30.
[4] R. Chen, R. Manochakian, L. James, A. G. Azzouqa, H. Shi, Y. Zhang, Y. Zhao, K. Zhou, Y. Lou, J Hematol Oncol 2020, 13, 58.
[5] V. Muthu, B. Mylliemngap, K. T. Prasad, D. Behera, N. Singh, Lung India 2019, 36, 32-37.
[6] S. Tan, D. Li, X. Zhu, Biomed Pharmacother 2020, 124, 109821.
[7] A. C. Tan, Thorac Cancer 2020, 11, 511-518.
[8] H. Hua, Q. Kong, H. Zhang, J. Wang, T. Luo, Y. Jiang, J Hematol Oncol 2019, 12, 71.
[9] N. Pallet, C. Legendre, Expert Opin Drug Saf 2013, 12, 177-186.
[10] aN. S. Gandhi, R. K. Tekade, M. B. Chougule, J Control Release 2014, 194, 238-256; bY. Hattori, M. Nakamura, N. Takeuchi, K. Tamaki, S. Shimizu, Y. Yoshiike, M. Taguchi, H. Ohno, K. I. Ozaki, H. Onishi, J Drug Target 2019, 27, 217-227.
[11] Y. S. Lee, S. W. Kim, J Control Release 2014, 190, 424-439.
[12] aJ. Ramos, T. Potta, O. Scheideler, K. Rege, ACS Appl Mater Interfaces 2014, 6, 14861-14873; bT. Potta, Z. Zhen, T. S. Grandhi, M. D. Christensen, J. Ramos, C. M. Breneman, K. Rege, Biomaterials 2014, 35, 1977-1988; cS. Goklany, P. Lu, S. Godeshala, A. Hall, E. Garrett-Mayer, C. Voelkel-Johnson, K. Rege, J Mater Chem B 2019, 7, 7014-7025; dS. Godeshala, B. Miryala, S. Dutta, M. D. Christensen, P. Nandi, P. L. Chiu, K. Rege, J Mater Chem B 2020, 8, 8558-8572.
[13] S. Kommareddy, M. Amiji, Bioconjug Chem 2005, 16, 1423-1432.
[14] N. S. Gandhi, S. Godeshala, D. T. Koomoa-Lange, B. Miryala, K. Rege, M. B. Chougule, Pharm Res 2018, 35, 188.
[15] H. H. Gustafson, D. Holt-Casper, D. W. Grainger, H. Ghandehari, Nano Today 2015, 10, 487-510.
[16] L. Shi, J. Zhang, M. Zhao, S. Tang, X. Cheng, W. Zhang, W. Li, X. Liu, H. Peng, Q. Wang, Nanoscale 2021, 13, 10748-10764.
[17] J. S. Suk, Q. Xu, N. Kim, J. Hanes, L. M. Ensign, Adv Drug Deliv Rev 2016, 99, 28-51.
[18] S. Sundaram, R. Trivedi, C. Durairaj, R. Ramesh, B. K. Ambati, U. B. Kompella, Clin Cancer Res 2009, 15, 7299-7308.
[19] X. Li, O. Taratula, O. Taratula, C. Schumann, T. Minko, Mini Rev Med Chem 2017, 17, 258-267.
[20] T. Minko, M. L. Patil, M. Zhang, J. J. Khandare, M. Saad, P. Chandna, O. Taratula, Methods Mol Biol 2010, 624, 281-294.
[21] aS. S. Dharap, Y. Wang, P. Chandna, J. J. Khandare, B. Qiu, S. Gunaseelan, P. J. Sinko, S. Stein, A. Farmanfarmaian, T. Minko, Proc Natl Acad Sci U S A 2005, 102, 12962-12967; bS. S. Dharap, B. Qiu, G. C. Williams, P. Sinko, S. Stein, T. Minko, J Control Release 2003, 91, 61-73.
[22] H. Gosnell, L. M. Kasman, T. Potta, L. Vu, E. Garrett-Mayer, K. Rege, C. Voelkel-Johnson, Journal of Controlled Release 2014, 176, 35-43.
[23] V. Vichai, K. Kirtikara, Nat Protoc 2006, 1, 1112-1116.
[24] aK. Hara, H. Tsujimoto, C. C. Huang, Y. Kawashima, R. Ando, O. Kusuoka, K. Tamura, M. Tsutsumi, J Toxicol Pathol 2012, 25, 19-26; bC. W. Liu, W. J. Lin, Int J Nanomedicine 2012, 7, 4749-4767.
[25] Q. Xu, L. M. Ensign, N. J. Boylan, A. Schon, X. Gong, J. C. Yang, N. W. Lamb, S. Cai, T. Yu, E. Freire, J. Hanes, ACS Nano 2015, 9, 9217-9227.
[26] C. G. Doss, S. Debottam, C. Debajyoti, Protoplasma 2013, 250, 787-792.
[27] E. Carrasco-Esteban, J. A. Domínguez-Rullán, P. Barrionuevo-Castillo, L. Pelari-Mici, O. Leaman, S. Sastre-Gallego, F. López-Campos, J Clin Transl Res 2021, 7, 140-155.
[28] P. Foroozandeh, A. A. Aziz, Nanoscale Res Lett 2018, 13, 339.
[29] T. S. Hauck, A. A. Ghazani, W. C. Chan, Small 2008, 4, 153-159.
[30] Y. W. Huang, M. Cambre, H. J. Lee, Int J Mol Sci 2017, 18.
[31] Y. Dai, X. Zhang, Macromol Biosci 2019, 19, e1800445.
[32] A. Hall, U. Lächelt, J. Bartek, E. Wagner, S. M. Moghimi, Mol Ther 2017, 25, 1476-1490.
[33] J. S. Suk, Q. Xu, N. Kim, J. Hanes, L. M. Ensign, Advanced drug delivery reviews 2016, 99, 28-51.
[34] aH. Y. Xue, S. Liu, H. L. Wong, Nanomedicine (Lond) 2014, 9, 295-312; bR. Bholakant, H. Qian, J. Zhang, X. Huang, D. Huang, J. Feijen, Y. Zhong, W. Chen, Biomacromolecules 2020, 21, 2966-2982.
[35] C. Li, H. Liu, Y. Sun, H. Wang, F. Guo, S. Rao, J. Deng, Y. Zhang, Y. Miao, C. Guo, J. Meng, X. Chen, L. Li, D. Li, H. Xu, H. Wang, B. Li, C. Jiang, J Mol Cell Biol 2009, 1, 37-45.
[36] aN. Man, Y. Chen, F. Zheng, W. Zhou, L. P. Wen, Autophagy 2010, 6, 449-454; bJ. Zhao, Z. Li, M. Wang, Z. Zhang, H. Ma, J. Chang, D. Gao, S. Wang, Acta Biochimica et Biophysica Sinica 2013, 45, 979-981.
[37] S. F. Dowdy, Nat Biotechnol 2017, 35, 222-229.
[38] K. Koushik, N. Bandi, S. Sundaram, U. B. Kompella, Pharm Res 2004, 21, 1034-1046.
[39] A. Sharma, D. Shambhwani, S. Pandey, J. Singh, H. Lalhlenmawia, M. Kumarasamy, S. K. Singh, D. K. Chellappan, G. Gupta, P. Prasher, K. Dua, D. Kumar, ACS Omega 2023, 8, 10-41.
[40] N. V. Nukolova, H. S. Oberoi, Y. Zhao, V. P. Chekhonin, A. V. Kabanov, T. K. Bronich, Mol Pharm 2013, 10, 3913-3921.
[41] H. Yang, S. Y. Fung, S. Xu, D. P. Sutherland, T. R. Kollmann, M. Liu, S. E. Turvey, ACS Nano 2015, 9, 6774-6784.
[42] C. Leuschner, C. S. Kumar, W. Hansel, W. Soboyejo, J. Zhou, J. Hormes, Breast Cancer Research and Treatment 2006, 99, 163-176.
[43] aJ. D. Obayemi, A. A. Salifu, S. C. Eluu, V. O. Uzonwanne, S. M. Jusu, C. C. Nwazojie, C. E. Onyekanne, O. Ojelabi, L. Payne, C. M. Moore, J. A. King, W. O. Soboyejo, Sci Rep 2020, 10, 8212; bM. S. Kim, S. Ma, A. Chelariu-Raicu, C. Leuschner, H. W. Alila, S. Lee, R. L. Coleman, A. K. Sood, Mol Cancer Ther 2020, 19, 2396-2406.
Acknowledgements: Dr. Rege is affiliated with Synergyan, LLC. Dr. Chougule is affiliated with Adexp Biopharm Consulting, Litigation and Patent Experts LLC.

Figure 1. In vitro cell viability of non-PEGylated mPAE (MP-NP), PEGylated mPAE (MP-PG-NP) and PEGylated, targeted mPAE (MP-PG-LR-NP) nanoparticles (without siRNA loaded) treated A549 and H460 cells at various concentrations (15µg/ml, 30 µg/ml, and 60 µg/ml).
