Preclinical, Clinical, and Translational Sciences
(W0930-10-54) Comparative In Vitro Release and Clinical Pharmacokinetics of Leuprorelin Depot 3.75mg, a One-Month Sustained-Release Formulation of Leuprorelin Acetate
Wednesday, October 23, 2024
9:30 AM - 10:30 AM MT
- JK
Jinho Kim, PhD
Researcher
Daewoong Pharmaceutical, Co., Ltd.
Yongin-Si, Kyonggi-do, Republic of Korea - JK
Jinho Kim, PhD
Researcher
Daewoong Pharmaceutical, Co., Ltd.
Yongin-Si, Kyonggi-do, Republic of Korea - GC
Gwangho Choo, MS
Researcher
Daewoong Pharmaceutical Co., Ltd.
Yongin-Si, Kyonggi-do, Republic of Korea - JK
Junsik Kim, MS
Team Leader
Daewoong Pharmaceutical Co., Ltd.
Yongin-Si, Kyonggi-do, Republic of Korea - GK
Gwanyoung Kim, Ph.D.
Head of Center
Daewoong Pharmaceutical Co., Ltd.
Yongin-Si, Kyonggi-do, Republic of Korea
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: We have introduced a new approach to microspheres development, demonstrating that this microsphere depot formulation exhibits characteristics equivalent to Leuplin Inj. 3.75mg (1), the first 1-month sustained-release microsphere product containing leuprorelin acetate.
Methods: The microsphere depot formulation, Leuprorelin Depot 3.75mg, was prepared aseptically by spray drying a solution of leuprorelin acetate and PLGA in glacial acetic acid, in an attempt to develop a new microsphere depot formulation that demonstrates in vivo release of leuprorelin over a 1-month period. Glacial acetic acid was used to achieve a homogeneous solution of drug and PLGA, and the spray-dried microspheres were washed in 1% PVA solution to remove residual acetic acid. In vitro characterization included determining particle size, drug encapsulation efficiency, and drug release profile. The pharmacokinetics of leuprorelin and the safety of the new formulation were investigated in a comparative human pharmacokinetics study following subcutaneous injection of an aqueous suspension of Leuprorelin Depot 3.75mg containing 3.75 mg of leuprorelin acetate. Results were compared with those for Leuplin Inj. 3.75mg.
Results: A 1-month microsphere depot formulation of leuprorelin acetate (Leuprorelin Depot 3.75mg), with a mean microsphere diameter of 18.2 μm, was prepared aseptically. This was achieved by spray drying a glacial acetic acid solution containing leuprorelin acetate and PLGA, followed by lyophilization in a D-mannitol solution. The drug loading content in Leuprorelin Depot 3.75mg was 8.50% (w/w), confirmed to be equivalent to that of Leuplin Inj. 3.75mg. In vitro studies demonstrated a substantially delayed long-term release of leuprorelin from the depot, showing a release profile similar to that of Leuplin Inj. 3.75mg (Figure 1) with a similarity factor (f2) of 78.0. The safety and pharmacokinetics of leuprorelin were evaluated over 28 days in a multi-center, randomized, single-dose, crossover study involving 33 healthy postmenopausal women who sequentially received subcutaneous injections of Leuprorelin Depot 3.75mg or Leuplin Inj. 3.75mg sequentially (3.75 mg leuprorelin acetate). Both formulations were well tolerated, and no serious adverse effects were observed during or after the study period. There were no significant differences in the maximum serum concentration (Cmax) and area under the curve (AUClast) of leuprorelin between the two formulations, as shown in Figure 2 and Table 1.
Conclusion: Using a proprietary spray-drying process, a PLGA microsphere depot formulation of leuprorelin acetate, Leuprorelin Depot 3.75mg, was successfully manufactured. The resulting microspheres are spherical in shape with a smooth surface, averaging approximately 18.2 μm in diameter. Leuprorelin Depot 3.75mg demonstrated significantly sustained in vitro release of leuprorelin over 28 days in a long-term test, showing comparability to Leuplin Inj. 3.75mg. In a human comparative pharmacokinetics study, Leuprorelin Depot 3.75mg maintained serum leuprorelin concentrations around 0.1 ng/mL for 28 days following subcutaneous administration to healthy postmenopausal women at a dose of 3.75 mg of leuprorelin acetate. Similar pharmacokinetic and safety profiles were observed with Leuplin Inj. 3.75mg, which was administered at the same leuprorelin acetate dose. Based on these findings, both formulations show comparable clinical efficacy and safety, suggesting they are clinically interchangeable. Therefore, Leuprorelin Depot 3.75mg, a 1-month sustained-release formulation of leuprorelin acetate, holds promise as an alternative medication option.
References: 1) Okada H, Inoue Y, Heya T, Ueno H, Toguchi H. Pharm Res 1991; 8:787-791
Acknowledgements: The authors declare no competing conflict of interest.
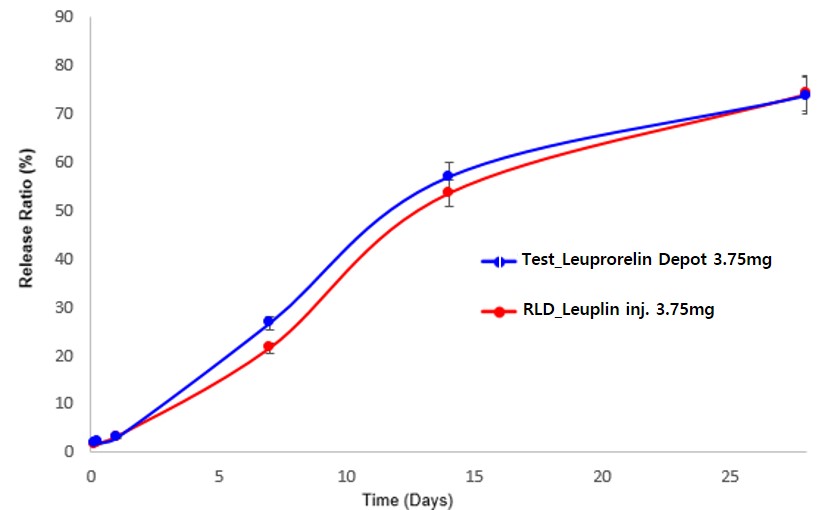 Figure 1. In vitro long-term release of leuprorelin from Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg (mean±SD, n=3).
Figure 1. In vitro long-term release of leuprorelin from Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg (mean±SD, n=3).
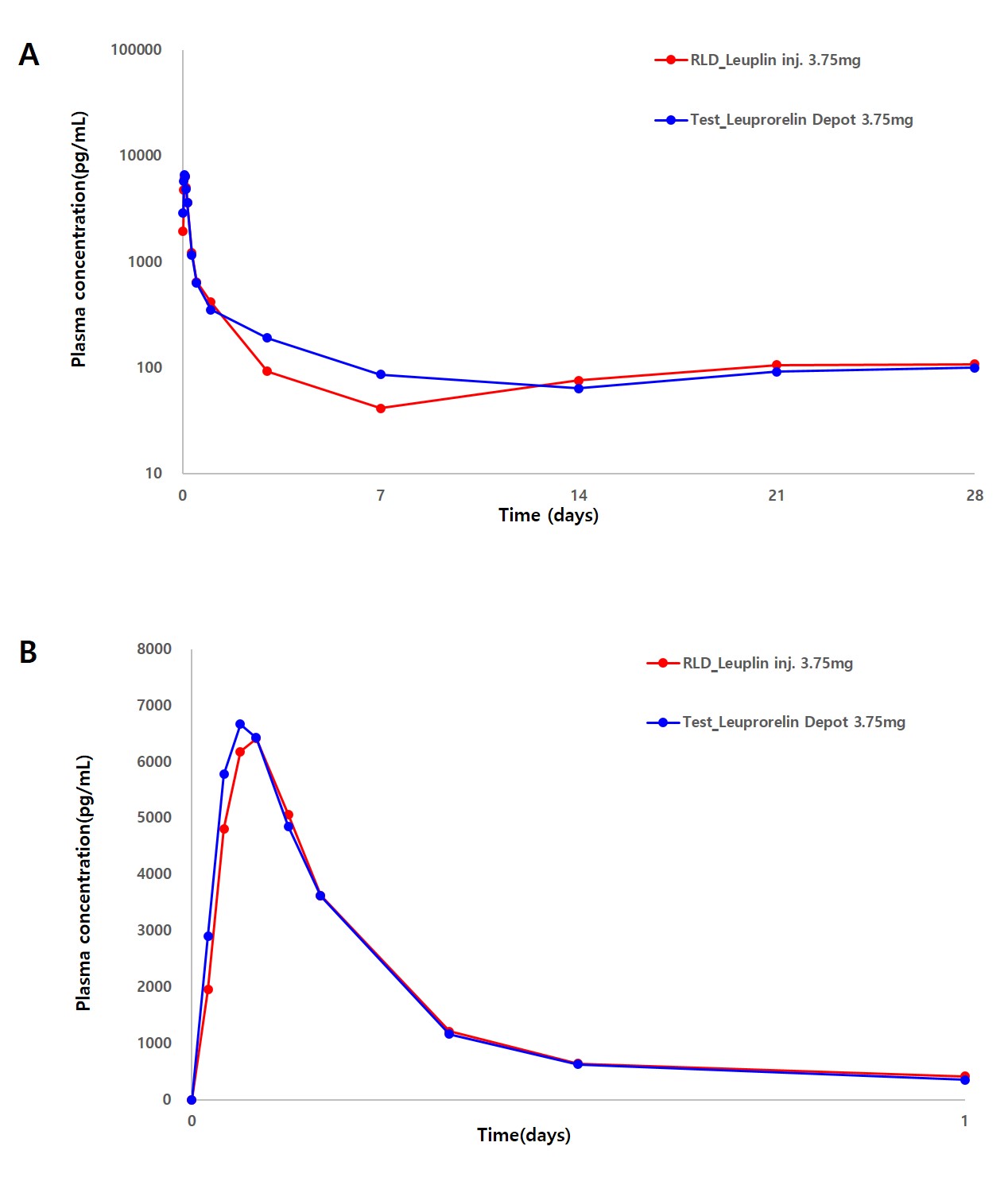 Figure 2. (A) Serum leuprorelin concentration-time profiles during a 28-day study period following subcutaneous administration of Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg to healthy postmenopausal women at a leuprolide acetate dose of 3.75 mg. (B) Serum Leuprorelin concentration-time profiles for 1 day were presented to compare the early burst of leuprorelin from the formulations.
Figure 2. (A) Serum leuprorelin concentration-time profiles during a 28-day study period following subcutaneous administration of Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg to healthy postmenopausal women at a leuprolide acetate dose of 3.75 mg. (B) Serum Leuprorelin concentration-time profiles for 1 day were presented to compare the early burst of leuprorelin from the formulations.
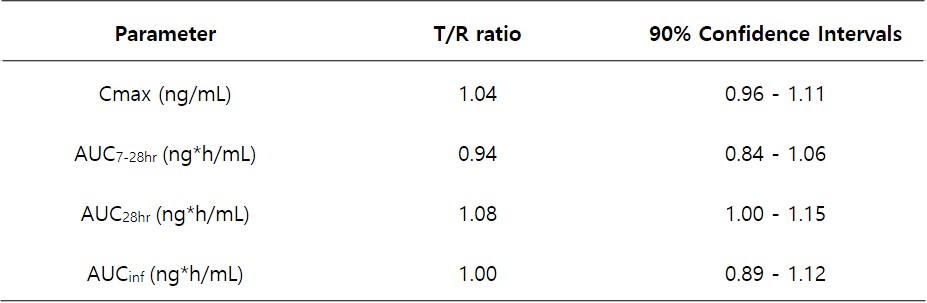 Table 1. Pharmacokinetic parameters of leuprorelin following a single subcutaneous injection of Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg were evaluated in 33 subjects receiving a dose of leuprorelin acetate at 3.75 mg.
Table 1. Pharmacokinetic parameters of leuprorelin following a single subcutaneous injection of Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg were evaluated in 33 subjects receiving a dose of leuprorelin acetate at 3.75 mg.
Methods: The microsphere depot formulation, Leuprorelin Depot 3.75mg, was prepared aseptically by spray drying a solution of leuprorelin acetate and PLGA in glacial acetic acid, in an attempt to develop a new microsphere depot formulation that demonstrates in vivo release of leuprorelin over a 1-month period. Glacial acetic acid was used to achieve a homogeneous solution of drug and PLGA, and the spray-dried microspheres were washed in 1% PVA solution to remove residual acetic acid. In vitro characterization included determining particle size, drug encapsulation efficiency, and drug release profile. The pharmacokinetics of leuprorelin and the safety of the new formulation were investigated in a comparative human pharmacokinetics study following subcutaneous injection of an aqueous suspension of Leuprorelin Depot 3.75mg containing 3.75 mg of leuprorelin acetate. Results were compared with those for Leuplin Inj. 3.75mg.
Results: A 1-month microsphere depot formulation of leuprorelin acetate (Leuprorelin Depot 3.75mg), with a mean microsphere diameter of 18.2 μm, was prepared aseptically. This was achieved by spray drying a glacial acetic acid solution containing leuprorelin acetate and PLGA, followed by lyophilization in a D-mannitol solution. The drug loading content in Leuprorelin Depot 3.75mg was 8.50% (w/w), confirmed to be equivalent to that of Leuplin Inj. 3.75mg. In vitro studies demonstrated a substantially delayed long-term release of leuprorelin from the depot, showing a release profile similar to that of Leuplin Inj. 3.75mg (Figure 1) with a similarity factor (f2) of 78.0. The safety and pharmacokinetics of leuprorelin were evaluated over 28 days in a multi-center, randomized, single-dose, crossover study involving 33 healthy postmenopausal women who sequentially received subcutaneous injections of Leuprorelin Depot 3.75mg or Leuplin Inj. 3.75mg sequentially (3.75 mg leuprorelin acetate). Both formulations were well tolerated, and no serious adverse effects were observed during or after the study period. There were no significant differences in the maximum serum concentration (Cmax) and area under the curve (AUClast) of leuprorelin between the two formulations, as shown in Figure 2 and Table 1.
Conclusion: Using a proprietary spray-drying process, a PLGA microsphere depot formulation of leuprorelin acetate, Leuprorelin Depot 3.75mg, was successfully manufactured. The resulting microspheres are spherical in shape with a smooth surface, averaging approximately 18.2 μm in diameter. Leuprorelin Depot 3.75mg demonstrated significantly sustained in vitro release of leuprorelin over 28 days in a long-term test, showing comparability to Leuplin Inj. 3.75mg. In a human comparative pharmacokinetics study, Leuprorelin Depot 3.75mg maintained serum leuprorelin concentrations around 0.1 ng/mL for 28 days following subcutaneous administration to healthy postmenopausal women at a dose of 3.75 mg of leuprorelin acetate. Similar pharmacokinetic and safety profiles were observed with Leuplin Inj. 3.75mg, which was administered at the same leuprorelin acetate dose. Based on these findings, both formulations show comparable clinical efficacy and safety, suggesting they are clinically interchangeable. Therefore, Leuprorelin Depot 3.75mg, a 1-month sustained-release formulation of leuprorelin acetate, holds promise as an alternative medication option.
References: 1) Okada H, Inoue Y, Heya T, Ueno H, Toguchi H. Pharm Res 1991; 8:787-791
Acknowledgements: The authors declare no competing conflict of interest.
 Figure 1. In vitro long-term release of leuprorelin from Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg (mean±SD, n=3).
Figure 1. In vitro long-term release of leuprorelin from Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg (mean±SD, n=3). Figure 2. (A) Serum leuprorelin concentration-time profiles during a 28-day study period following subcutaneous administration of Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg to healthy postmenopausal women at a leuprolide acetate dose of 3.75 mg. (B) Serum Leuprorelin concentration-time profiles for 1 day were presented to compare the early burst of leuprorelin from the formulations.
Figure 2. (A) Serum leuprorelin concentration-time profiles during a 28-day study period following subcutaneous administration of Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg to healthy postmenopausal women at a leuprolide acetate dose of 3.75 mg. (B) Serum Leuprorelin concentration-time profiles for 1 day were presented to compare the early burst of leuprorelin from the formulations.  Table 1. Pharmacokinetic parameters of leuprorelin following a single subcutaneous injection of Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg were evaluated in 33 subjects receiving a dose of leuprorelin acetate at 3.75 mg.
Table 1. Pharmacokinetic parameters of leuprorelin following a single subcutaneous injection of Leuprorelin Depot 3.75mg and Leuplin Inj. 3.75mg were evaluated in 33 subjects receiving a dose of leuprorelin acetate at 3.75 mg.