Bioanalytics - Biomolecular
(W1030-06-31) Comparison of the Performance of Luminex-Based and MSD-Based Immunogenicity Assays for Multivalent HPV Vaccines
Wednesday, October 23, 2024
10:30 AM - 11:30 AM MT
- XN
Xin Ning, PhD
Senior Scientist I
WuXi AppTec Co., Ltd.
Shanghai, Shanghai, China (People's Republic) - XW
Xuefang Wang, BS
Director I
WuXi AppTec Co., Ltd.
Shanghai, Shanghai, China (People's Republic) - XN
Xin Ning, PhD
Senior Scientist I
WuXi AppTec Co., Ltd.
Shanghai, Shanghai, China (People's Republic) - PL
Peipei Liang, MS
Group Leader I
WuXi AppTec Co., Ltd.
Shanghai, Shanghai, China (People's Republic) - CZ
Chenpu Zhang, MS
Senior Director
WuXi AppTec Co., Ltd.
Shanghai, Shanghai, China (People's Republic) - LL
Lan Li, MS
Vice President
WuXi AppTec Co., Ltd.
Shanghai, Shanghai, China (People's Republic)
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Human papillomavirus (HPV) causes a variety of diseases with low or high risks in humans. Over 100 different types of HPVs have been identified and molecularly characterized[1]. Studies on immunogenicity towards various HPVs help to develop multivalent HPV vaccines to build immune barriers. It is known that pseudovirus-based neutralizing assay (PBNA), treated as the “golden standard” for detecting anti-HPV antibodies for evaluating the immunogenicity of vaccine candidates, has some weaknesses, such as low efficiency and throughput[2]. Aiming to these issues, this study aims to develop two direct-binding-IgG assays, proven to be acceptable surrogates for the PBNA in measuring vaccine responses[3-5], with higher efficiency and throughput by using Luminex and Meso Scale Discovery (MSD) instrument to quantitatively detect total IgG for the evaluation of immunogenicity towards multivalent HPV vaccines.
Methods: For the Luminex-based assay, four different magnetic beads are labeled separately with one of the four HPV subsets (HPV16, 18, 6, 11) Virus-like Particles (VLPs), correspondingly. Following that, unknown samples are incubated with the mixture of 4 beads so that anti-HPV 16/18/11/6 VLP antibodies in the unknown samples are captured onto the corresponding beads. PE-labeled Goat F(ab’)2 Anti-Human (IgG Fc antibody) is then added and incubated. Finally, the fluorescence signal is read using a Luminex instrument. For the MSD-based assay, four (4) types of streptavidin (SA)-labeled U-PLEX linkers are combined with four biotin-labeled HPV subsets (HPV16, 18, 6, 11), correspondingly. Following the specific binding between the four SA linkers and their corresponding receptors in wells, the different HPV subset proteins are immobilized to their corresponding places on the plate. Samples containing human Anti-HPV IgG are then added into the wells and incubated. Ruthenium-labeled Anti-human IgG antibody is added into the wells afterward. After adding the appropriate read buffer, the plate is read by MSD to get the analytical results.
Results: Compared with PBNA, for both Luminex and MSD assays, the total time of a single run can be reduced from forty-eight hours[6] to six hours, a reduction of 700%. Furthermore, the results of total IgG corresponding to the four HPV subtypes from one sample could be obtained simultaneously by using each of these two assays, rather than only a single subtype at one time by the PBNA test, indicating the ability to detect multi-HPV subtype with 4-fold higher throughput. Validation parameters were assessed, including accuracy, precision, dilution linearity, selectivity, specificity, stability, and dynamic ranges. The bias of the quality control samples (QCs) against theoretical values for both assays was no more than 23.5%, indicating that the Luminex-based assay and the MSD-based assay both demonstrated reliability. To further support that, 86 unknown single human serum samples were analyzed separately by the Luminex and MSD assays. The total IgG values measured for the same subtypes of the samples from the two assays showed that the difference was less than 30%, demonstrating consistency between the two assays. Among the comparisons of validation parameters between these two assays, differences were observed in specificity and dynamic ranges. The specificity of multi-target assays could affect the accuracy of a single-target analysis in the presence of cross-interferences such as HPV16, 18, 6, and 11 VLPs in this case. For the Luminex-based assay, QCs’ bias was less than 25% when concentrations of HPV6 and HPV11 VLPs in samples were less than 1.0 µg/mL and 0.8 µg/mL, correspondingly. For the MSD-based assay, QCs’ bias analysis showed that when concentrations of HPV6 and HPV11 VLPs in samples were more than 0.6 µg/mL and 0.5 µg/mL, the bias could correspondingly be more than 25%. The cross-interference of HPV6 and HPV11 VLPs in the MSD-based assay is 40% more significant than in the Luminex assay. In comparing assay dynamic range, it is found Luminex reached assay range at 0.0447 to 10.9 U/ml for HPV6, 0.077 to 18.8 U/ml for HPV11, 0.0912 to 22.3 U/mL for HPV 16 and 0.0275 to 6.72 U/mL for HPV 18, while MSD reached at 0.0323 to 16.2 U/ml for HPV6, 0.0500 to 25.0 U/ml for HPV11, 0.0459 to 23.0 U/mL for HPV 16 and 0.0187 to 9.35 U/mL for HPV 18.
Conclusion: MSD-based and Luminex-based immunogenicity assays for multivalent HPV Vaccines were successfully developed and validated. Compared with the PBNA, the Luminex and MSD assays showed advantages by multi-HPV subtype detection in one assay and 700% total assay time reduction, respectively, leading to higher assay efficiency and throughput. Both assays showed good accuracy, and the total IgG test results from both assays are consistent with one another. However, they also showed their own advantages, such as better specificity performance of Luminex and wider dynamic ranges of the MSD assay. Based on the needs for clinical trials, one could choose either Luminex or MSD for robust assay performance with higher assay efficiency and throughput in detecting total anti-HPV antibodies.
References: [1] Guidelines to Assure the Quality, Safety and Efficacy of Recombinant Human Papillomavirus Virus-Like Particle Vaccines, WHO/BS/06.2050 - Final
[2] Recommendations to assure the quality, safety and efficacy of recombinant human papillomavirus virus-like particle vaccines, Replacement of Annex 1 of WHO Technical Report Series, No. 962
[3] Correlation between ELISA and pseudovirion-based neutralisation assay for detecting antibodies against human papillomavirus acquired by natural infection or by vaccination, Human Vaccines & Immunotherapeutics 10:3, 740–746
[4] Correlation between direct ELISA, single epitope-based inhibition ELISA and Pseudovirionbased neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine, Human Vaccines, 4:6, 425-434
[5] Peptide enzyme-linked immunosorbent assay (pELISA) as a possible alternative to the neutralization test for evaluating the immune response to IBV vaccine, BMC Veterinary Research (2021) 17:51
[6] Pseudotype Neutralization Assays: From Laboratory Bench to Data Analysis, Methods and Protoc. 2018, 1, 8
Acknowledgements: N/A
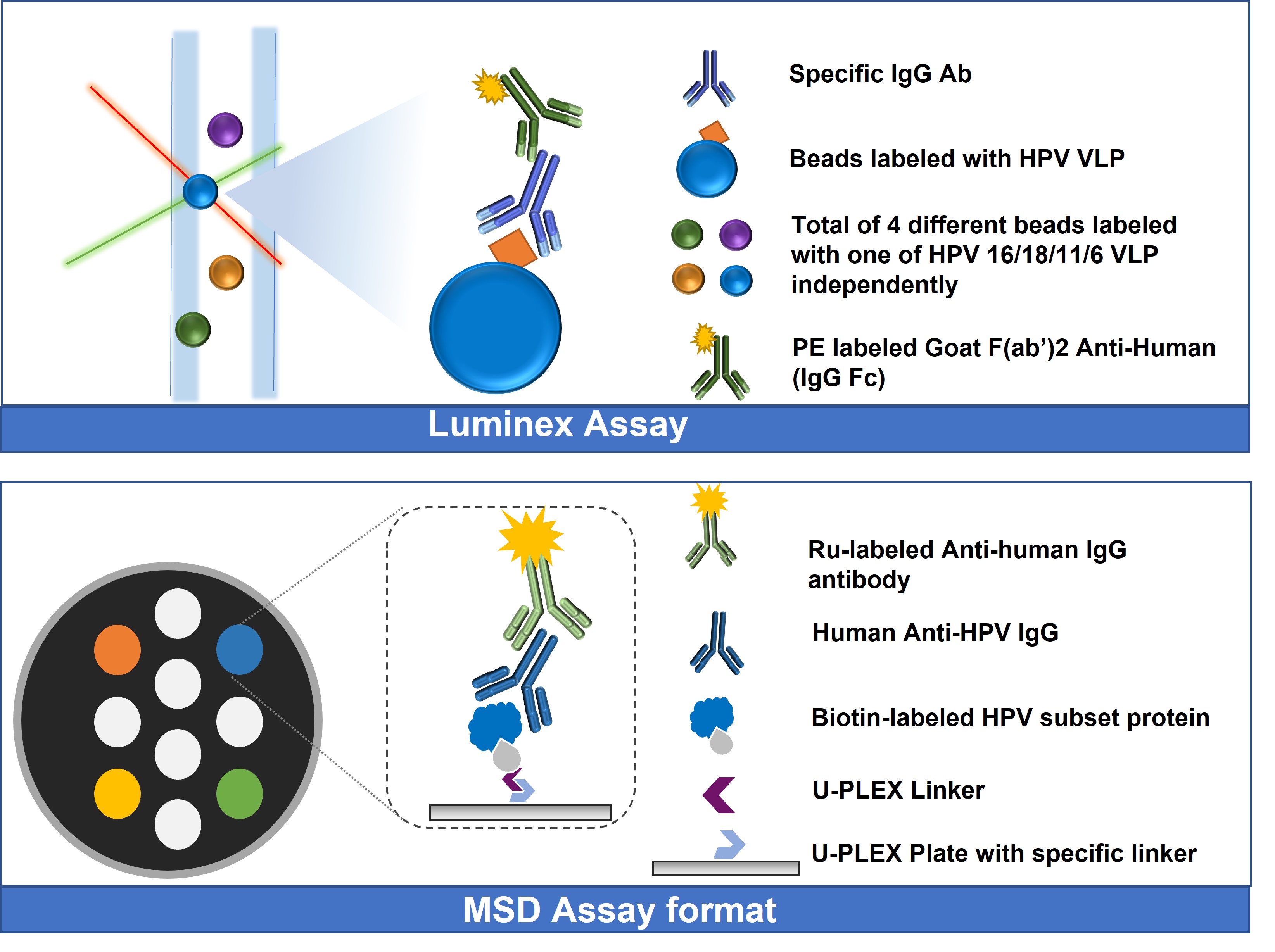 Figure 1. Schemes of Luminex-based (upper) and MSD-based (lower) immunogenicity assays for multivalent HPV vaccines
Figure 1. Schemes of Luminex-based (upper) and MSD-based (lower) immunogenicity assays for multivalent HPV vaccines
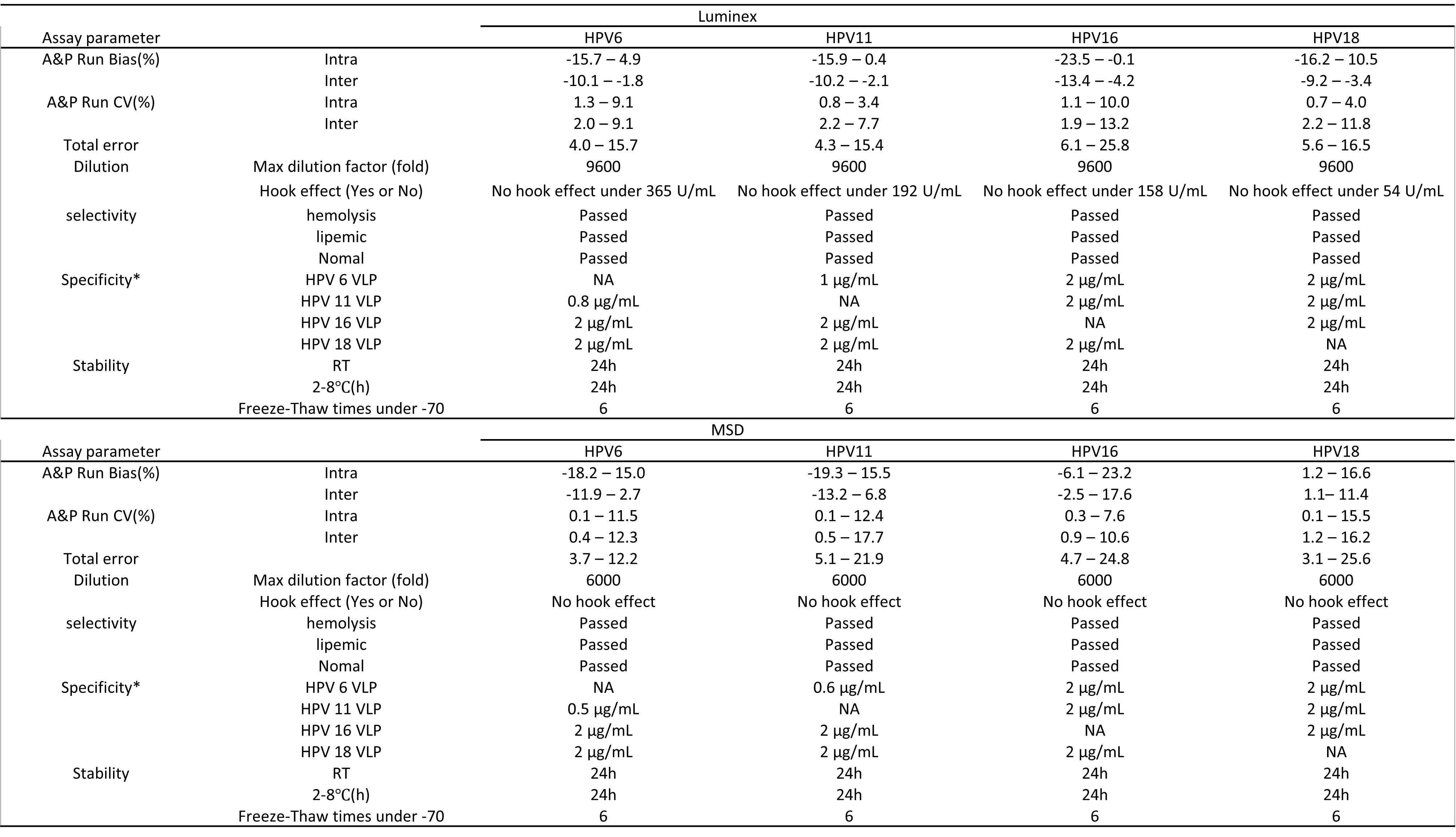 Figure 2. Summary of validation parameters for Luminex-based (upper) and MSD-based (lower) immunogenicity assays for multivalent HPV vaccines. *The max concentrations of HPV VLPs that cause no interference are exhibited here to demonstrate the specificity of two assays.
Figure 2. Summary of validation parameters for Luminex-based (upper) and MSD-based (lower) immunogenicity assays for multivalent HPV vaccines. *The max concentrations of HPV VLPs that cause no interference are exhibited here to demonstrate the specificity of two assays.
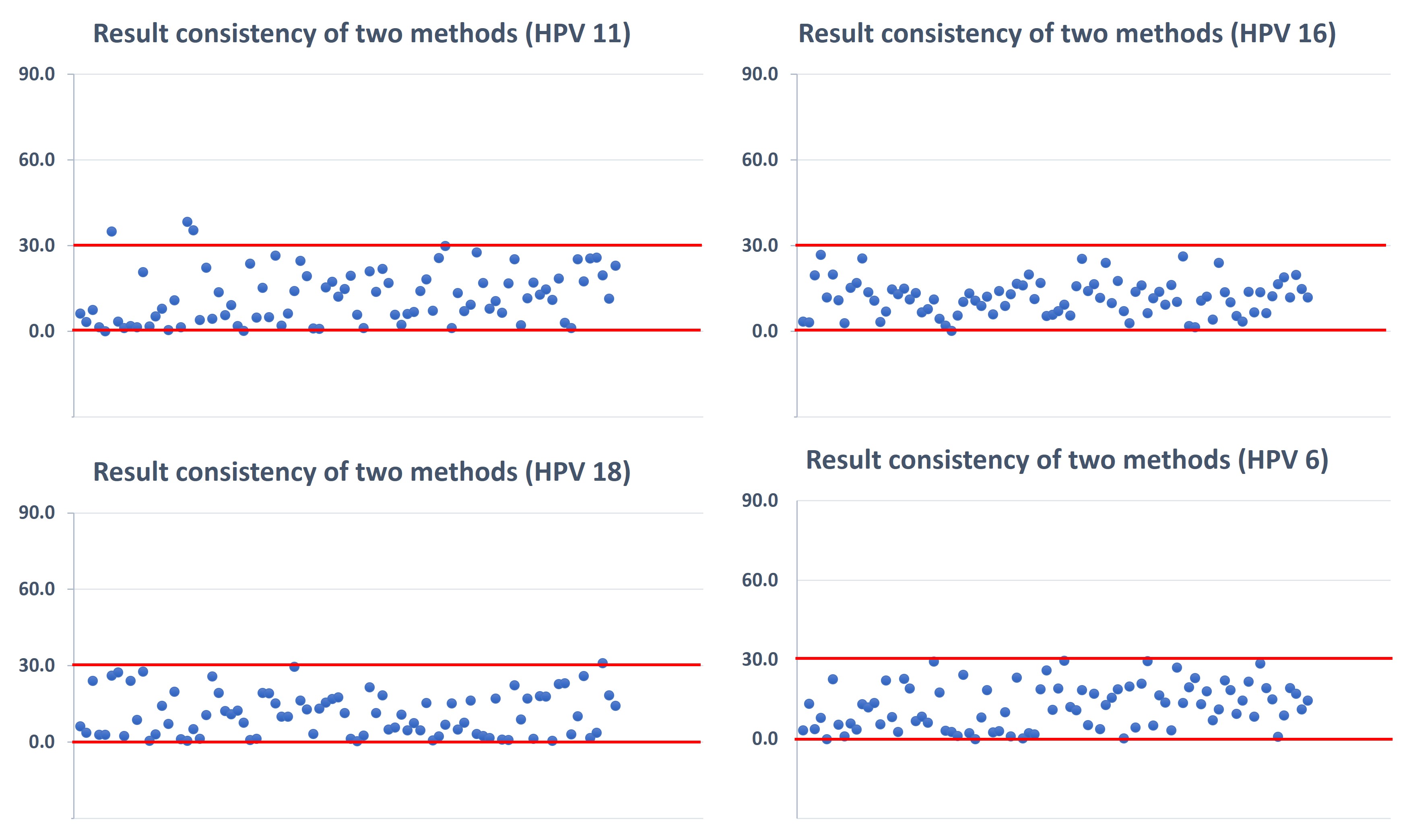 Figure 3. Result consistency of Luminex-based and MSD-based immunogenicity assays for multivalent HPV vaccines, demonstrated through the comparative data of four HPV subtypes. Comparative data are calculated using the formula as 2*(c2 - c1) / (c2 + c1), in which c2 is the concentration of corresponding HPV subtypes detected by MSD and c1 is the concentration of corresponding HPV subtypes detected by Luminex. The absolute values of the calculated results are used in statistics.
Figure 3. Result consistency of Luminex-based and MSD-based immunogenicity assays for multivalent HPV vaccines, demonstrated through the comparative data of four HPV subtypes. Comparative data are calculated using the formula as 2*(c2 - c1) / (c2 + c1), in which c2 is the concentration of corresponding HPV subtypes detected by MSD and c1 is the concentration of corresponding HPV subtypes detected by Luminex. The absolute values of the calculated results are used in statistics.
Methods: For the Luminex-based assay, four different magnetic beads are labeled separately with one of the four HPV subsets (HPV16, 18, 6, 11) Virus-like Particles (VLPs), correspondingly. Following that, unknown samples are incubated with the mixture of 4 beads so that anti-HPV 16/18/11/6 VLP antibodies in the unknown samples are captured onto the corresponding beads. PE-labeled Goat F(ab’)2 Anti-Human (IgG Fc antibody) is then added and incubated. Finally, the fluorescence signal is read using a Luminex instrument. For the MSD-based assay, four (4) types of streptavidin (SA)-labeled U-PLEX linkers are combined with four biotin-labeled HPV subsets (HPV16, 18, 6, 11), correspondingly. Following the specific binding between the four SA linkers and their corresponding receptors in wells, the different HPV subset proteins are immobilized to their corresponding places on the plate. Samples containing human Anti-HPV IgG are then added into the wells and incubated. Ruthenium-labeled Anti-human IgG antibody is added into the wells afterward. After adding the appropriate read buffer, the plate is read by MSD to get the analytical results.
Results: Compared with PBNA, for both Luminex and MSD assays, the total time of a single run can be reduced from forty-eight hours[6] to six hours, a reduction of 700%. Furthermore, the results of total IgG corresponding to the four HPV subtypes from one sample could be obtained simultaneously by using each of these two assays, rather than only a single subtype at one time by the PBNA test, indicating the ability to detect multi-HPV subtype with 4-fold higher throughput. Validation parameters were assessed, including accuracy, precision, dilution linearity, selectivity, specificity, stability, and dynamic ranges. The bias of the quality control samples (QCs) against theoretical values for both assays was no more than 23.5%, indicating that the Luminex-based assay and the MSD-based assay both demonstrated reliability. To further support that, 86 unknown single human serum samples were analyzed separately by the Luminex and MSD assays. The total IgG values measured for the same subtypes of the samples from the two assays showed that the difference was less than 30%, demonstrating consistency between the two assays. Among the comparisons of validation parameters between these two assays, differences were observed in specificity and dynamic ranges. The specificity of multi-target assays could affect the accuracy of a single-target analysis in the presence of cross-interferences such as HPV16, 18, 6, and 11 VLPs in this case. For the Luminex-based assay, QCs’ bias was less than 25% when concentrations of HPV6 and HPV11 VLPs in samples were less than 1.0 µg/mL and 0.8 µg/mL, correspondingly. For the MSD-based assay, QCs’ bias analysis showed that when concentrations of HPV6 and HPV11 VLPs in samples were more than 0.6 µg/mL and 0.5 µg/mL, the bias could correspondingly be more than 25%. The cross-interference of HPV6 and HPV11 VLPs in the MSD-based assay is 40% more significant than in the Luminex assay. In comparing assay dynamic range, it is found Luminex reached assay range at 0.0447 to 10.9 U/ml for HPV6, 0.077 to 18.8 U/ml for HPV11, 0.0912 to 22.3 U/mL for HPV 16 and 0.0275 to 6.72 U/mL for HPV 18, while MSD reached at 0.0323 to 16.2 U/ml for HPV6, 0.0500 to 25.0 U/ml for HPV11, 0.0459 to 23.0 U/mL for HPV 16 and 0.0187 to 9.35 U/mL for HPV 18.
Conclusion: MSD-based and Luminex-based immunogenicity assays for multivalent HPV Vaccines were successfully developed and validated. Compared with the PBNA, the Luminex and MSD assays showed advantages by multi-HPV subtype detection in one assay and 700% total assay time reduction, respectively, leading to higher assay efficiency and throughput. Both assays showed good accuracy, and the total IgG test results from both assays are consistent with one another. However, they also showed their own advantages, such as better specificity performance of Luminex and wider dynamic ranges of the MSD assay. Based on the needs for clinical trials, one could choose either Luminex or MSD for robust assay performance with higher assay efficiency and throughput in detecting total anti-HPV antibodies.
References: [1] Guidelines to Assure the Quality, Safety and Efficacy of Recombinant Human Papillomavirus Virus-Like Particle Vaccines, WHO/BS/06.2050 - Final
[2] Recommendations to assure the quality, safety and efficacy of recombinant human papillomavirus virus-like particle vaccines, Replacement of Annex 1 of WHO Technical Report Series, No. 962
[3] Correlation between ELISA and pseudovirion-based neutralisation assay for detecting antibodies against human papillomavirus acquired by natural infection or by vaccination, Human Vaccines & Immunotherapeutics 10:3, 740–746
[4] Correlation between direct ELISA, single epitope-based inhibition ELISA and Pseudovirionbased neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine, Human Vaccines, 4:6, 425-434
[5] Peptide enzyme-linked immunosorbent assay (pELISA) as a possible alternative to the neutralization test for evaluating the immune response to IBV vaccine, BMC Veterinary Research (2021) 17:51
[6] Pseudotype Neutralization Assays: From Laboratory Bench to Data Analysis, Methods and Protoc. 2018, 1, 8
Acknowledgements: N/A
 Figure 1. Schemes of Luminex-based (upper) and MSD-based (lower) immunogenicity assays for multivalent HPV vaccines
Figure 1. Schemes of Luminex-based (upper) and MSD-based (lower) immunogenicity assays for multivalent HPV vaccines Figure 2. Summary of validation parameters for Luminex-based (upper) and MSD-based (lower) immunogenicity assays for multivalent HPV vaccines. *The max concentrations of HPV VLPs that cause no interference are exhibited here to demonstrate the specificity of two assays.
Figure 2. Summary of validation parameters for Luminex-based (upper) and MSD-based (lower) immunogenicity assays for multivalent HPV vaccines. *The max concentrations of HPV VLPs that cause no interference are exhibited here to demonstrate the specificity of two assays.  Figure 3. Result consistency of Luminex-based and MSD-based immunogenicity assays for multivalent HPV vaccines, demonstrated through the comparative data of four HPV subtypes. Comparative data are calculated using the formula as 2*(c2 - c1) / (c2 + c1), in which c2 is the concentration of corresponding HPV subtypes detected by MSD and c1 is the concentration of corresponding HPV subtypes detected by Luminex. The absolute values of the calculated results are used in statistics.
Figure 3. Result consistency of Luminex-based and MSD-based immunogenicity assays for multivalent HPV vaccines, demonstrated through the comparative data of four HPV subtypes. Comparative data are calculated using the formula as 2*(c2 - c1) / (c2 + c1), in which c2 is the concentration of corresponding HPV subtypes detected by MSD and c1 is the concentration of corresponding HPV subtypes detected by Luminex. The absolute values of the calculated results are used in statistics. 