Formulation and Delivery - Biomolecular
(W1030-07-41) Phage-Antibiotic Loaded Coaxial Electrospun Fibres for Antibiotic Resistant Wound Infections
Wednesday, October 23, 2024
10:30 AM - 11:30 AM MT
- TJ
Tian Ju, Ph.D.
PhD Student
University College London
London, England, United Kingdom - TJ
Tian Ju, Ph.D.
PhD student
University College London
London, England, United Kingdom
Presenting Author(s)
Main Author(s)
Purpose: Antibacterial resistance is a major global public health challenge, associated with an estimated 4.95 million deaths in 2019. Multidrug resistant P. aeruginosa is detected in many infected wounds and is very challenging to treat with antibiotics. An alternative to antibiotics is to use bacteriophages, highly specific viruses able to kill even resistant bacteria. This work uses electrospinning technology to incorporate two anti-pseudomonas aeruginosa phages in the core and ciprofloxacin in the shell of the fibres for treating antibiotic resistant wound infections. Phages were blended with polyvinyl alcohol (PVA) solution. PVA/water solution does not use organic solvents and thus maintains infectivity of the phages. Ciprofloxacin was mixed with polyvinylpyrrolidone (PVP) and PVP/ethyl cellulose (EC) shell solutions. The drug release can be controlled by varying components of the shell.
Methods: Electrospinning: coaxial electrospinning. Core: PVA, shell: PVP and PVP/ethyl cellylose. Phage morphology: Double layered agar assays, TEM. Phage viability, stability and phage release from fibres: double layered agar assays. Antibacterial efficacy of Phage-antibiotic combination: broth dilution methods and isothermal calorimetry. Material characterization: TEM, SEM, DSC, XRD, FTIR. Drug release and encapsulation efficiency: UV spectrometry
Results: Two anti-P. aeruginosa phages were utilized in this work. PA01 exhibited a capsid with mean diameter of 68 nm. No obvious tail can be seen. PA02 is a filamentous phage. PA01 and PA02. Isothermal calorimetry and broth dilution methods revealed different inhibiting mechanism of PA01 and PA02 against P. aeruginosa. PA01 cannot delay the growth of bacteria in the beginning but can reduce bacterial burden after 24 h-incubation. PA02 can delay the growth of bacteria for 4 h but cannot reduce bacterial burden. Antibacterial efficacy of Phage-antibiotic combination: PA01 and PA02 decreases MIC of ciprofloxacin to ½ MIC against P. aeruginosa. Phage and ciprofloxacin demonstrated additive effect. Phage-antibiotic loaded electrospun fibres was examined by isothermal calorimetry. The results presented phages-antibiotic loaded fibres exhibited similar antibacterial efficacy as adding ciprofloxacin and phage lysate directly, indicating fibres successfully encapsulated the phages without affecting their antibacterial efficacy. Fibre morphology. Electrospun fibres: TEM images showed clear core-shell structure of fibres. Viability and Stability of phages. Viability of phages in the fibres: The viability of two phages in the fibres is 47%. Stability of phages in the fibres: The phage titres do not show significant loss after 6 months of storage at -20 °C. 1 log loss of titres was found after 6 months of storage at 4 °C. Phage and drug release. Varying proportion of PVP/EC is the shell can control ciprofloxacin release profiles. Pure PVP shell presented the highest release rate: 90% of drug was released within 8 h. PVP/EC 8/2 shell has the lowest drug release rate with 50% of drug released within 8 h. PVP/EC 9/1 shell has the moderate drug release rate, which 60% of drug was released within 8 hours. The increased proportion of EC in the fibres leads to the decreased drug release rate. However, different shell components do not have significant effect on phage release. Phage release profiles for PVP and PVP/EC 82 shell demonstrated very similar trends: most phages were released within 2 h. Further investigation will be done for PVP/EC 91 shell.
Conclusion: This study explored the possibilities of using phage-antibiotic loaded electrospun fibres for treating wounds infected with P. aeruginosa. The viability of phages in the fibres is 47%. Stability tests indicated storing the fibres at -20 °C resulted in minimal titre loss after 6 months of storage. 1 log titre loss was detected after the phage loaded fibres were stored at 4 °C. The drug release can be controlled via varying shell components. PVP shell demonstrated highest drug release rate, while PVP/EC 8/2 extended drug release up to 48 h. Phage release was not affected by the different components of shell. Phages and ciprofloxacin demonstrated additive effect against P. aeruginosa. The antibacterial activity of the loaded fibres was studied using isothermal calorimetry and found to be retained after electrospinning. Thus, phage loaded electrospun fibres have potential for the development of wound dressings for treating antibiotic resistant infections.
.jpg) Morphology of PA01 and PA02 phages and phage-antibiotic loaded electrospun fibres
Morphology of PA01 and PA02 phages and phage-antibiotic loaded electrospun fibres
.jpg) Drug/Phage release from coaxial fibres with different shell components
Drug/Phage release from coaxial fibres with different shell components
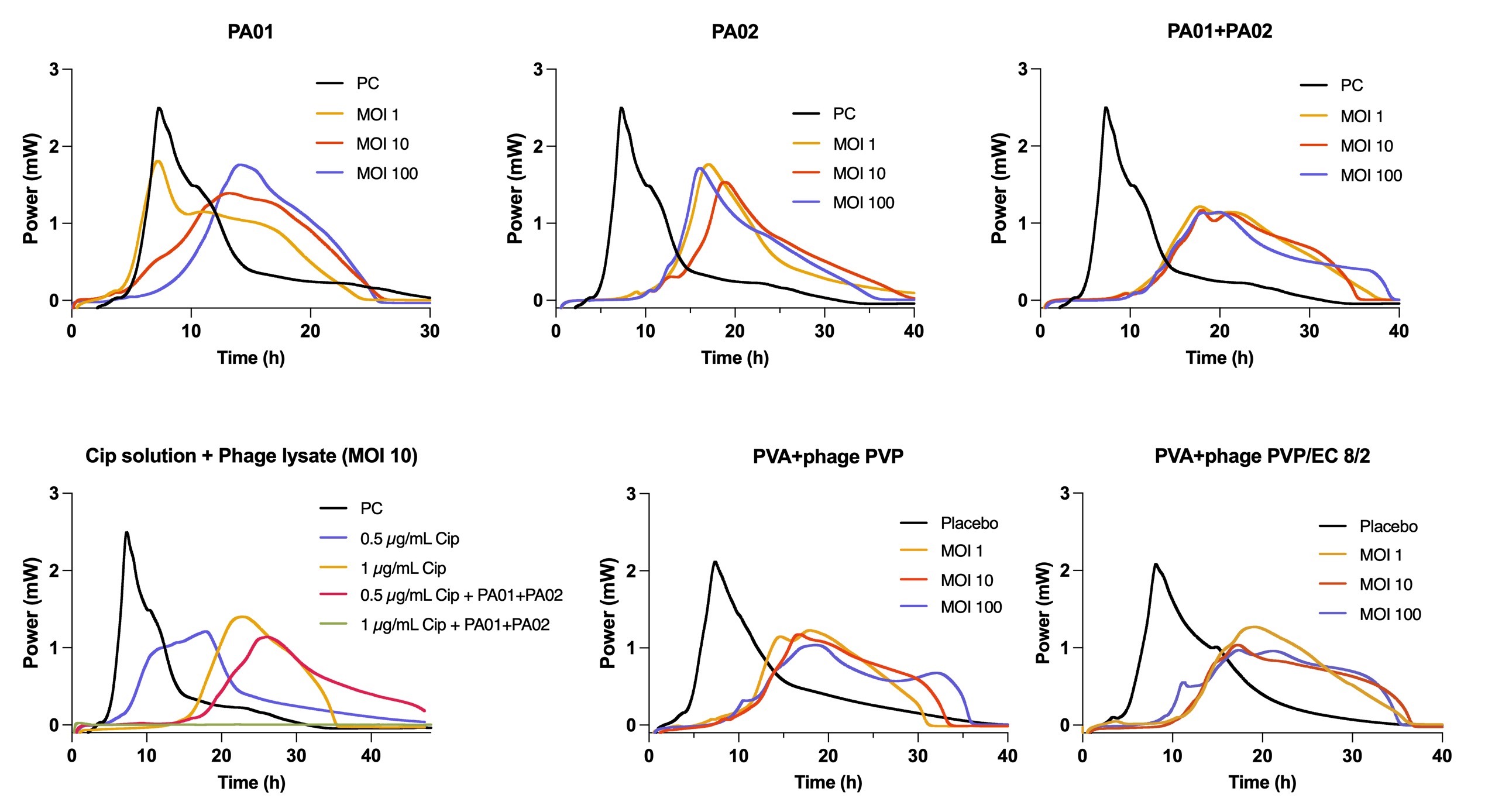 Calorimetric growth curves for P. aeruginosa in the presence of PA01, PA02, PA01&PA02 combination, phage-antibiotic combination and phage loaded fibres.
Calorimetric growth curves for P. aeruginosa in the presence of PA01, PA02, PA01&PA02 combination, phage-antibiotic combination and phage loaded fibres.
Methods: Electrospinning: coaxial electrospinning. Core: PVA, shell: PVP and PVP/ethyl cellylose. Phage morphology: Double layered agar assays, TEM. Phage viability, stability and phage release from fibres: double layered agar assays. Antibacterial efficacy of Phage-antibiotic combination: broth dilution methods and isothermal calorimetry. Material characterization: TEM, SEM, DSC, XRD, FTIR. Drug release and encapsulation efficiency: UV spectrometry
Results: Two anti-P. aeruginosa phages were utilized in this work. PA01 exhibited a capsid with mean diameter of 68 nm. No obvious tail can be seen. PA02 is a filamentous phage. PA01 and PA02. Isothermal calorimetry and broth dilution methods revealed different inhibiting mechanism of PA01 and PA02 against P. aeruginosa. PA01 cannot delay the growth of bacteria in the beginning but can reduce bacterial burden after 24 h-incubation. PA02 can delay the growth of bacteria for 4 h but cannot reduce bacterial burden. Antibacterial efficacy of Phage-antibiotic combination: PA01 and PA02 decreases MIC of ciprofloxacin to ½ MIC against P. aeruginosa. Phage and ciprofloxacin demonstrated additive effect. Phage-antibiotic loaded electrospun fibres was examined by isothermal calorimetry. The results presented phages-antibiotic loaded fibres exhibited similar antibacterial efficacy as adding ciprofloxacin and phage lysate directly, indicating fibres successfully encapsulated the phages without affecting their antibacterial efficacy. Fibre morphology. Electrospun fibres: TEM images showed clear core-shell structure of fibres. Viability and Stability of phages. Viability of phages in the fibres: The viability of two phages in the fibres is 47%. Stability of phages in the fibres: The phage titres do not show significant loss after 6 months of storage at -20 °C. 1 log loss of titres was found after 6 months of storage at 4 °C. Phage and drug release. Varying proportion of PVP/EC is the shell can control ciprofloxacin release profiles. Pure PVP shell presented the highest release rate: 90% of drug was released within 8 h. PVP/EC 8/2 shell has the lowest drug release rate with 50% of drug released within 8 h. PVP/EC 9/1 shell has the moderate drug release rate, which 60% of drug was released within 8 hours. The increased proportion of EC in the fibres leads to the decreased drug release rate. However, different shell components do not have significant effect on phage release. Phage release profiles for PVP and PVP/EC 82 shell demonstrated very similar trends: most phages were released within 2 h. Further investigation will be done for PVP/EC 91 shell.
Conclusion: This study explored the possibilities of using phage-antibiotic loaded electrospun fibres for treating wounds infected with P. aeruginosa. The viability of phages in the fibres is 47%. Stability tests indicated storing the fibres at -20 °C resulted in minimal titre loss after 6 months of storage. 1 log titre loss was detected after the phage loaded fibres were stored at 4 °C. The drug release can be controlled via varying shell components. PVP shell demonstrated highest drug release rate, while PVP/EC 8/2 extended drug release up to 48 h. Phage release was not affected by the different components of shell. Phages and ciprofloxacin demonstrated additive effect against P. aeruginosa. The antibacterial activity of the loaded fibres was studied using isothermal calorimetry and found to be retained after electrospinning. Thus, phage loaded electrospun fibres have potential for the development of wound dressings for treating antibiotic resistant infections.
.jpg) Morphology of PA01 and PA02 phages and phage-antibiotic loaded electrospun fibres
Morphology of PA01 and PA02 phages and phage-antibiotic loaded electrospun fibres.jpg) Drug/Phage release from coaxial fibres with different shell components
Drug/Phage release from coaxial fibres with different shell components Calorimetric growth curves for P. aeruginosa in the presence of PA01, PA02, PA01&PA02 combination, phage-antibiotic combination and phage loaded fibres.
Calorimetric growth curves for P. aeruginosa in the presence of PA01, PA02, PA01&PA02 combination, phage-antibiotic combination and phage loaded fibres.