Formulation and Delivery - Biomolecular
(W1130-07-37) Drug Product Development Approaches to Overcome Stability Challenges of Antibody Drug Conjugates (ADCs)
- NJ
Nishant K. Jain, Ph.D.
Senior Principal Scientist
Bristol-Myers Squibb Company
New Brunswick, New Jersey, United States - NJ
Nishant K. Jain, Ph.D.
Senior Principal Scientist
Bristol-Myers Squibb Company
New Brunswick, New Jersey, United States - AB
Ashwin Basarkar, Ph.D.
Associate Director
Bristol-Myers Squibb Company
New Brunswick, New Jersey, United States - JH
Jordan Hirschman, MS
Scientist
Bristol-Myers Squibb Company
New Brunswick, New Jersey, United States - SZ
Sherry Zhu, Ph.D.
Principal Scientist
Bristol-Myers Squibb Company
New Brunswick, New Jersey, United States - BD
Barton Dear, Ph.D.
Principal Scientist
Bristol-Myers Squibb Company
New Brunswick, New Jersey, United States - NT
Nicole Taylor, Ph.D.
Principal Scientist
Bristol-Myers Squibb Company
New Brunswick, New Jersey, United States - CE
Christina Evans, MS
Scientist
Bristol-Myers Squibb Company
New Brunswick, New Jersey, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: The temperature-dependent reversible phase transition behavior of the drug substance intermediate was characterized by exposing the unconjugated antibody to temperatures ranging from 5 to 25°C. Various physicochemical properties, including appearance, size, viscosity, and molecular interactions, were analyzed. The impact of different excipients on the colloidal properties of the unconjugated antibody was then monitored to identify optimal formulation conditions to prevent phase transition. In the second case study, an ADC, with and without varying levels of formulation excipients, was subjected to shear and interfacial stresses through agitation and other in-house developed tools. Physical stability was assessed by measuring optical density and sub-visible particulates. An optimal formulation was selected, and manufacturing process parameters, such as filling and mixing speeds, were optimized to improve the physical stability of the drug product.
Results: The unconjugated antibody formed a gel-like structure when exposed to refrigerated temperatures but returned to a solution phase at ambient conditions. Diverse types of colloidal interactions were observed in various buffer systems and pH environments, correlating well with the viscosity and gel-forming propensity of the unconjugated antibody at cold temperatures. Further optimization of the selected buffer/pH conditions, in combination with an additional excipient, resulted in a formulation that exhibited overall repulsive interactions and did not exhibit the reversible phase transition behavior of the unconjugated antibody. In the second case study, the ADC, when subjected to agitation in the base formulation, showed a substantial increase in subvisible particles and turbidity. These issues were reduced significantly upon optimizing the formulation conditions. Further characterization of the impact of manufacturing-relevant stress conditions indicated that the ADC is sensitive to both interfacial and shear stresses. Optimization of the process parameters to reduce these stresses proved beneficial. The filling and mixing parameters were optimized, resulting in an ADC drug product with good long-term stability.
Conclusion: These examples highlight the unique challenges that emerging complex modalities, such as ADCs, present during product development. However, they also demonstrate how our robust scientific approaches and rational drug product design strategies can effectively address these challenges. By leveraging a deep understanding of physicochemical properties and optimizing formulation and process parameters, we can ensure the optimal stability and efficacy of these advanced therapeutics.
.jpg) Figure 1: Illustration of temperature dependent reversable phase transition of unconjugated antibody, and its mitigation through formulation optimization.
Figure 1: Illustration of temperature dependent reversable phase transition of unconjugated antibody, and its mitigation through formulation optimization.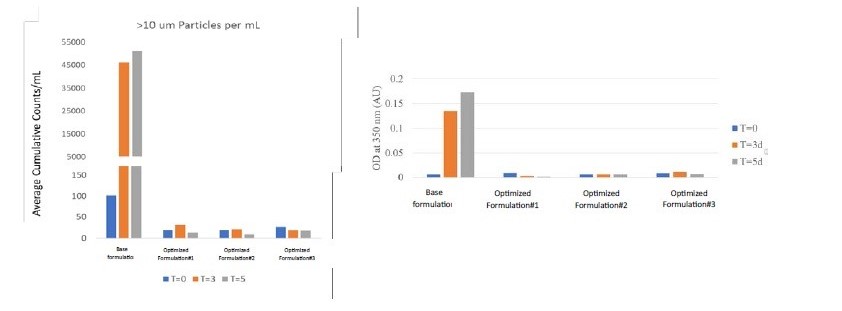 Improved physical stability of ADC upon optimization of formulation conditions. All the samples were subjected to agitation stress for up to 5 days.
Improved physical stability of ADC upon optimization of formulation conditions. All the samples were subjected to agitation stress for up to 5 days.