Formulation and Delivery - Chemical
(W1230-07-39) Lipid Nanoparticles Particle Size Control: Effect of Buffer and Linear vs Circular mRNA

Raquib Hasan, PhD
Senior Scientist, Advanced Technologies
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States
Raquib Hasan, PhD
Senior Scientist, Advanced Technologies
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States- AK
Aashna Khan, MS
Co-Op
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States - EC
Eric Crampon, Ph.D.
Associate Director, Potency Assay CoE, Vaccines
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States - VY
Volkan Yesilyurt, Ph.D.
Senior Scientist, Advanced Technologies, Drug Product and Device Development
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States - VG
Venkat Garigapati, Ph.D.
Senior Scientific Fellow, Drug Product Development
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States - VK
Victus Kordorwu, MS
Co-Op
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: LNPs were manufactured using NanoAssemblr Ignite microfluidic system. Different aqueous to lipid phase ratios were used for manufacturing LNPs. Buffer strengths were changed as well as mRNAs (circular vs linear). mRNA concentration, ionizable lipid to mRNA ratio, lipid molar ratios were kept constant in all formulations. LNPs were dialyzed against before characterization. Malvern zetasizer was used for measuring particles size and ribogreen assay was used for encapsulation efficiency measurement.
Results: Decreasing aqueous phase ratio relative to ethanol phase ratio led to larger particle sizes. Incubating larger particles, prepared at aqueous: lipid ratio of 1.5:1 before dialyzing lead to further increase in particle sizes which was not the case for smaller particles prepared at aqueous: lipid ratio of 3:1. Further decreasing aqueous phase to 1:1 (aqueous: lipid) lead to higher polydispersity (figure 1). Circular mRNA showed better encapsulation efficiency in larger particles compared to when linear mRNA was used at different buffer strengths. This phenomenon was observed with different sized circular mRNA where size varied by ~1.8x kb (figure 1, 2 and 3) Changing buffer strength led to higher encapsulation efficiency in larger particles noticeably matching encapsulation of mRNA in larger particles to the smaller particles (figure 1). 8x scaled up LNPs showed similar properties as small scale LNPs. Particle size and encapsulation efficiency were preserved throughout further processing of particles i.e. dialysis and concentration (figure 3).
Conclusion: In the study, we developed a method for LNP particle size control. Circular mRNA seemed to have more robustness in terms of particle size control, showed higher encapsulation efficiency and was less susceptible to buffer changes.
References: [1] K. J. Hassett et al., “Impact of lipid nanoparticle size on mRNA vaccine immunogenicity,” Journal of Controlled Release, vol. 335, pp. 237–246, Jul. 2021, doi: 10.1016/j.jconrel.2021.05.021.
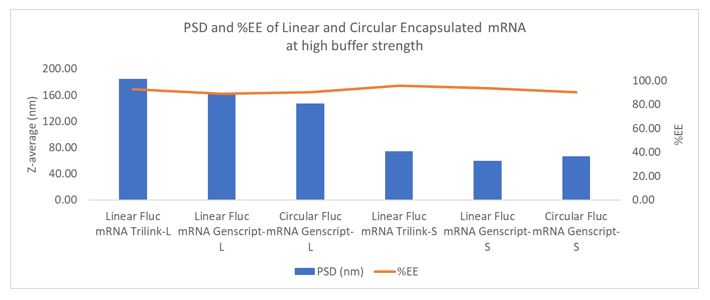 Figure 2: Comparison between linear vs circular mRNA at higher buffer strength. Target PSD was achieved with high encapsulation efficiency.
Figure 2: Comparison between linear vs circular mRNA at higher buffer strength. Target PSD was achieved with high encapsulation efficiency.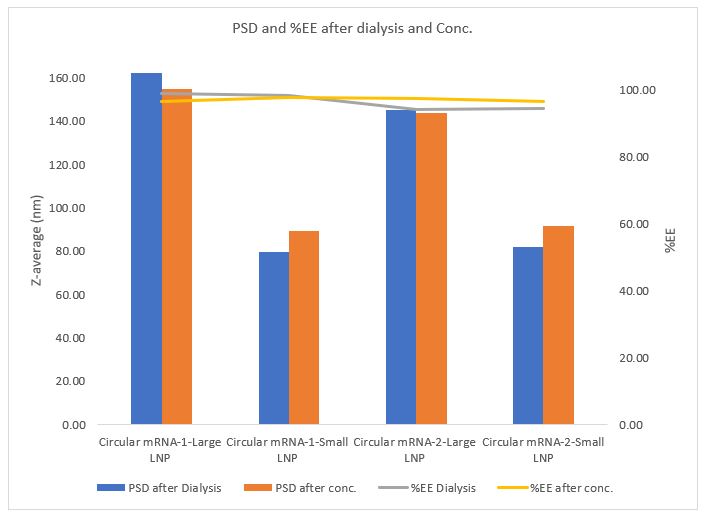 Figure 3: Large circular mRNA showed similar particle size control with great encapsulation efficiency at scale up. PSD and %EE was maintained throughout the processing steps.
Figure 3: Large circular mRNA showed similar particle size control with great encapsulation efficiency at scale up. PSD and %EE was maintained throughout the processing steps.