Manufacturing and Analytical Characterization - Chemical
(T1130-10-54) Developing Robust Control Strategies for an Unexpected Fluorination Process Investigation
Tuesday, October 22, 2024
11:30 AM - 12:30 PM MT
- RG
Rekha Gangam, Ph.D.
Associate Principal Scientist
Merck & Co., Inc.
Totowa, New Jersey, United States - BK
Brittany Kassim, Ph.D.
Director
Merck & Co., Inc.
Rahway, New Jersey, United States
Presenting Author(s)
Main Author(s)
Purpose: During the belzutifan fluorination process, the widely used fluorinating agent Selectfluor was employed to convert hydroxy indanone (pre-penultimate) into fluorodiol intermediate, which is a penultimate compound. However, the process development phase revealed variations in reaction performance and unexpected corrosion of the Hastelloy reactor. The presence of fluoride ions in Selectfluor necessitates the use of corrosion-resistant metals such as Hastelloy, which is a nickel metal that is alloyed with molybdenum and chromium. Detailed analytical characterization revealed that the corrosion problem primarily stemmed from higher levels of chloride present in the incoming materials. Additionally, elevated levels of chlorinated impurities were detected in both the penultimate compound and the belzutifan active pharmaceutical ingredient (API). A comprehensive investigation was conducted to identify potential sources of incoming chloride, and it was determined that variable levels of chloride were present in both the selector and the hydroxy indanone starting material. This indicated the likelihood of chloride contamination from multiple sources throughout the process. Effective control strategies were implemented to address the issue and ensure consistent performance of the process.
Methods: To monitor chloride levels in the incoming raw material Selectfluor and the hydroxy indanone starting material, an ion chromatographic method was developed. The method utilized a Dionex lonPac column AS19, 4 µm, and a guard column AG19, 4 µm, with a flow rate of 0.4 mL/min. A KOH concentration gradient ranging from 2-50 mM over a run time of 25 minutes was used. The method was validated with a limit of detection of 50 ppm and a limit of quantitation of 100 ppm. The linearity of the method was established over a range of 100-2000 ppm of the target sample concentration. Sample solutions were prepared at a concentration of 2 mg/mL, and commercially available chloride standards were prepared at a concentration of 1000 ppm. Additionally, to measure the corrosion level, a Hastelloy coupon was immersed in the reaction vessel, and the weight loss of the coupon was measured as an indicator of corrosion.
Results: The initial analytical investigation aimed to compare an underperforming vendor (vendor-2) lot with a well-performing vendor (vendor-1) lot to identify any distinguishing characteristics that could explain the specific vendor-2 lot's underperformance. Ion chromatographic analysis revealed variations in chloride levels between the two vendors, which correlated with differences in corrosion levels (refer to Table 1). Chloride spiking experiments were conducted, and the resulting chlorinated impurity levels and corrosion were measured. The downstream fate and purge of chlorinated impurities were also determined.
Conclusion: Based on the observed correlation between chloride levels and corrosion, as well as the formation of chlorinated impurities, the chloride levels were targeted to control to ≤1000 ppm in both Selectfluor and hydroxy indanone.
References: An Investigation into the Unexpected Corrosion of Nickel Alloy Vessels with Selectfluor
Organic Process Research & Development 2022 26 (1), 159-164
Acknowledgements: Wenjun Liu, Stephen Dalby, Tao Wang, Nastaran Salehi and Michael Pirnot
 Figure 1: The observed corrosion of Hastelloy coupon with two different vendor’s selectfluor material
Figure 1: The observed corrosion of Hastelloy coupon with two different vendor’s selectfluor material
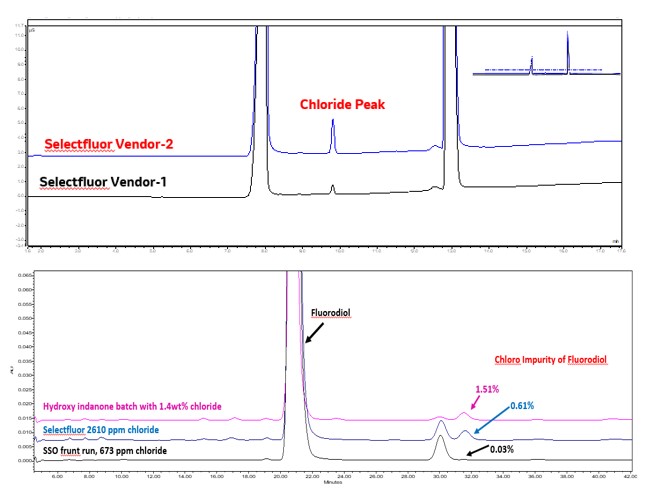 Figure 2: Overlay of Ion Chromatography chromatograms of two different vendor's selectfluor material and an overlay of fluorodiol impurity profile chromatograms with various levels of incoming chloride levels
Figure 2: Overlay of Ion Chromatography chromatograms of two different vendor's selectfluor material and an overlay of fluorodiol impurity profile chromatograms with various levels of incoming chloride levels
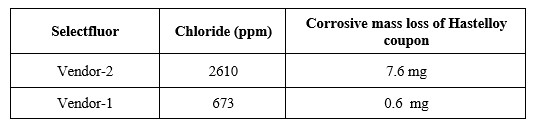 Table 1: Chloride Content of two different vendors’ selectfluor material and corresponding corrosive mass loss of the Hastelloy coupon
Table 1: Chloride Content of two different vendors’ selectfluor material and corresponding corrosive mass loss of the Hastelloy coupon
Methods: To monitor chloride levels in the incoming raw material Selectfluor and the hydroxy indanone starting material, an ion chromatographic method was developed. The method utilized a Dionex lonPac column AS19, 4 µm, and a guard column AG19, 4 µm, with a flow rate of 0.4 mL/min. A KOH concentration gradient ranging from 2-50 mM over a run time of 25 minutes was used. The method was validated with a limit of detection of 50 ppm and a limit of quantitation of 100 ppm. The linearity of the method was established over a range of 100-2000 ppm of the target sample concentration. Sample solutions were prepared at a concentration of 2 mg/mL, and commercially available chloride standards were prepared at a concentration of 1000 ppm. Additionally, to measure the corrosion level, a Hastelloy coupon was immersed in the reaction vessel, and the weight loss of the coupon was measured as an indicator of corrosion.
Results: The initial analytical investigation aimed to compare an underperforming vendor (vendor-2) lot with a well-performing vendor (vendor-1) lot to identify any distinguishing characteristics that could explain the specific vendor-2 lot's underperformance. Ion chromatographic analysis revealed variations in chloride levels between the two vendors, which correlated with differences in corrosion levels (refer to Table 1). Chloride spiking experiments were conducted, and the resulting chlorinated impurity levels and corrosion were measured. The downstream fate and purge of chlorinated impurities were also determined.
Conclusion: Based on the observed correlation between chloride levels and corrosion, as well as the formation of chlorinated impurities, the chloride levels were targeted to control to ≤1000 ppm in both Selectfluor and hydroxy indanone.
References: An Investigation into the Unexpected Corrosion of Nickel Alloy Vessels with Selectfluor
Organic Process Research & Development 2022 26 (1), 159-164
Acknowledgements: Wenjun Liu, Stephen Dalby, Tao Wang, Nastaran Salehi and Michael Pirnot
 Figure 1: The observed corrosion of Hastelloy coupon with two different vendor’s selectfluor material
Figure 1: The observed corrosion of Hastelloy coupon with two different vendor’s selectfluor material  Figure 2: Overlay of Ion Chromatography chromatograms of two different vendor's selectfluor material and an overlay of fluorodiol impurity profile chromatograms with various levels of incoming chloride levels
Figure 2: Overlay of Ion Chromatography chromatograms of two different vendor's selectfluor material and an overlay of fluorodiol impurity profile chromatograms with various levels of incoming chloride levels Table 1: Chloride Content of two different vendors’ selectfluor material and corresponding corrosive mass loss of the Hastelloy coupon
Table 1: Chloride Content of two different vendors’ selectfluor material and corresponding corrosive mass loss of the Hastelloy coupon