Formulation and Delivery - Chemical
(T1130-09-53) Smart Formulating of Pharmaceutical Emulsions Using HLD-NAC Theory
Tuesday, October 22, 2024
11:30 AM - 12:30 PM MT
- CB
Connor Bilchak, Ph.D.
Scientist
BASF Corporation
Tarrytown, New York, United States - CB
Connor Bilchak, Ph.D.
Scientist
BASF Corporation
Tarrytown, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: Pharmaceutical Emulsions are complex mixtures of lipophilic and hydrophilic excipients, compatibilized with emulsifiers. Basic design principles, such as Hydrophile-Lipophile Balance (HLB) have previously been used to guide choice of excipients and remain the gold standard for formulating; however, most suffer from fundamental knowledge gaps that limit their accuracy; for example, HLB values cannot be directly measured, and the effects of other physio-chemical parameters of the emulsion (e.g., salinity, temperature, and concentration effects) are ignored. This work demonstrates the application of hydrophile-lipophile difference / net-average curvature theory (HLD-NAC) to the formulation emulsions using pharmaceutical excipients. HLD-NAC permits accurate prediction of emulsion stability, as well as minimum droplet size, interfacial tension, and shelf life, using easy-to-obtain oil/surfactant physiochemical properties. The HLD equation for nonionic surfactants is1: HLD = 0.13S-0.16EACN + 0.06 (T-25) +Cc. Where S is the formulation salinity, EACN is the effective alkane carbon number of the oil, T is temperature, and Cc is the surfactant characteristic curvature; Cc may also be concentration dependent. HLD values < 0 indicate oil-in-water emulsions, while values >0 indicate water-in-oil emulsions; the greater the magnitude of the HLD value, the more stable the emulsion is. HLD is often combined with Net-Average-Curvature (NAC) theory, which relates the net curvature, Hnet, of the emulsion interface to HLD value and the surfactant dimensions2: Hnet = 1/Roil - 1/Rwater = -HLD/Lt. Where Roil are the radii of solubilized oil and water droplets, and Lt is the emulsifier tail length. Given a calculated HLD and an estimate of Lt, HLD-NAC gives the radius of a solubilized oil or water droplet, which in turn can be used in a predictive capacity. Characteristic values of BASF Kolliphor® emulsifiers were measured using salinity scans. A fixed mass of surfactant was added to 6mL of water with varying salinities (often 10-25 mg/100ml). An equivalent volume of a linear alkane with known EACN (C6-C12) was added and the resulting solution was thoroughly mixed and equilibrated in an incubation chamber. Phase volumes were determined using image analysis of the scans. Surfactant head group areas were calculated from surface tension measurements. Tail lengths were calculated based on molecular structure using the modified Tanford equation.3
Results: The emulsifier Cc is a critical parameter for employing HLD-NAC theory. Values generally range from -3 (for O/W emulsifiers) and +3 (for W/O emulsions). Figure 1A shows a representative salinity scan of Kolliphor® PS60 in hexane; the optimal salinity S* occurs in vial #2. The Cc is determined from S* using equation (1) and setting HLD=0. Performing salinity scans at different temperatures or using oils with various EACNs can improve the accuracy of the Cc measurement; here, Cc is determined to within ±0.1. HLD-NAC theory can also be used to estimate the phase volumes for any given emulsion; predictions for the water-emulsion-oil phase boundaries are shown as dashed lines in Figure 1, while the points indicate the actual phase boundaries; there is very good agreement between theory and experiment. Figure 1B shows the concentration dependence of Cc for an O/W and W/O emulsifier; the Cc magnitude for both decreases at higher concentrations, indicating a decrease in the solubilization capacity of the emulsifier (i.e., the well-known ‘over-formulating’ effect). This effect is importantly not captured by HLB theory but is adequately incorporated into HLD-NAC. The results from HLD-NAC can be used in a predictive capacity. For example, Figure 2 compares the emulsion droplet diameters measured using optical microscopy to the Hinze-Kolmogorov3 equation for turbulent mixers—using predictions for the oil/water interfacial tension from HLD-NAC— and finds good agreement between theory and experiment. Finally, comparing HLD-NAC stability predictions with those of HLB (Table I) shows much better alignment with observed formulation stabilities. HLD-NAC theory therefore improves on the existing concepts of HLB using only Equation 1.
Conclusion: Existing concepts of HLD-NAC theory were employed in a series of benchmarking studies for developing pharmaceutical emulsions. The relevant material parameters are accurately measured using simple scanning experiments, and values are in alignment with empirical observations. Extensions of the theory can be used to determine a myriad of emulsion properties, including interfacial tension, droplet size, and shelf life. Application of HLD-NAC as a predictive tool using pharmaceutical excipients can allow for rational design of emulsion-based products, virtually eliminating the need for lengthy product development and troubleshooting.
References: 1. Salager, Prog Colloid Polym Sci, 1996
2. Acosta, Szekeres, et al., Langmuir, 2003
3. Tanford, J. Phys. Chem, 1972
4. Kiran & Acosta, I&EC Research, 2015
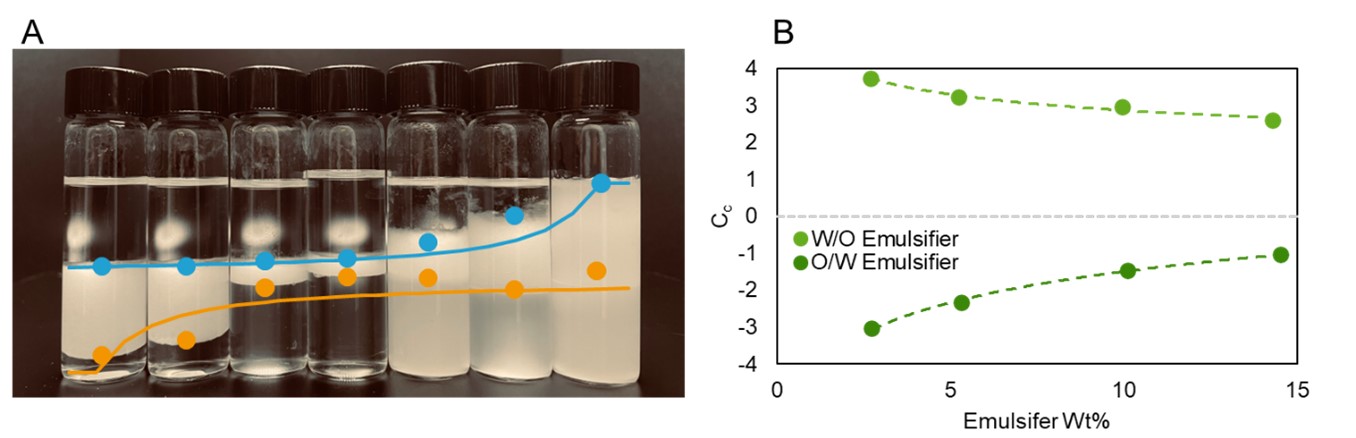 (A) Salinity Scan of Kolliphor® PS60 in hexane. (B) concentration dependence of the Cc value for an O/W and W/O emulsifier.
(A) Salinity Scan of Kolliphor® PS60 in hexane. (B) concentration dependence of the Cc value for an O/W and W/O emulsifier.
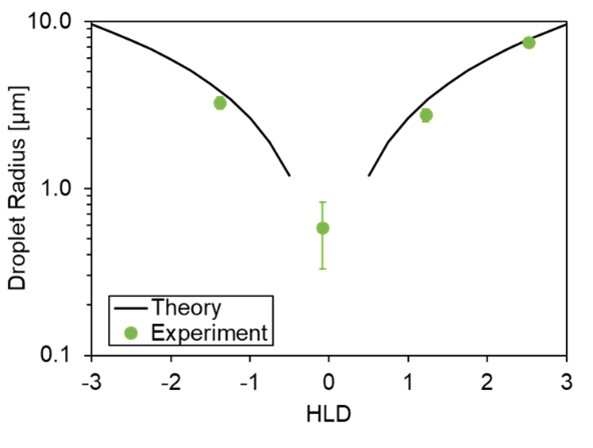 Experimental emulsion droplet radii from the salinity scan shown in Figure 1 compared to predicted values using HLD-NAC Theory
Experimental emulsion droplet radii from the salinity scan shown in Figure 1 compared to predicted values using HLD-NAC Theory
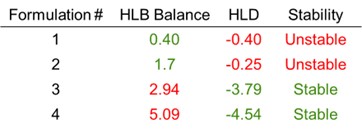 Comparison of observed and predicted stability for four representative pharmaceutical emulsions.
Comparison of observed and predicted stability for four representative pharmaceutical emulsions.
Results: The emulsifier Cc is a critical parameter for employing HLD-NAC theory. Values generally range from -3 (for O/W emulsifiers) and +3 (for W/O emulsions). Figure 1A shows a representative salinity scan of Kolliphor® PS60 in hexane; the optimal salinity S* occurs in vial #2. The Cc is determined from S* using equation (1) and setting HLD=0. Performing salinity scans at different temperatures or using oils with various EACNs can improve the accuracy of the Cc measurement; here, Cc is determined to within ±0.1. HLD-NAC theory can also be used to estimate the phase volumes for any given emulsion; predictions for the water-emulsion-oil phase boundaries are shown as dashed lines in Figure 1, while the points indicate the actual phase boundaries; there is very good agreement between theory and experiment. Figure 1B shows the concentration dependence of Cc for an O/W and W/O emulsifier; the Cc magnitude for both decreases at higher concentrations, indicating a decrease in the solubilization capacity of the emulsifier (i.e., the well-known ‘over-formulating’ effect). This effect is importantly not captured by HLB theory but is adequately incorporated into HLD-NAC. The results from HLD-NAC can be used in a predictive capacity. For example, Figure 2 compares the emulsion droplet diameters measured using optical microscopy to the Hinze-Kolmogorov3 equation for turbulent mixers—using predictions for the oil/water interfacial tension from HLD-NAC— and finds good agreement between theory and experiment. Finally, comparing HLD-NAC stability predictions with those of HLB (Table I) shows much better alignment with observed formulation stabilities. HLD-NAC theory therefore improves on the existing concepts of HLB using only Equation 1.
Conclusion: Existing concepts of HLD-NAC theory were employed in a series of benchmarking studies for developing pharmaceutical emulsions. The relevant material parameters are accurately measured using simple scanning experiments, and values are in alignment with empirical observations. Extensions of the theory can be used to determine a myriad of emulsion properties, including interfacial tension, droplet size, and shelf life. Application of HLD-NAC as a predictive tool using pharmaceutical excipients can allow for rational design of emulsion-based products, virtually eliminating the need for lengthy product development and troubleshooting.
References: 1. Salager, Prog Colloid Polym Sci, 1996
2. Acosta, Szekeres, et al., Langmuir, 2003
3. Tanford, J. Phys. Chem, 1972
4. Kiran & Acosta, I&EC Research, 2015
 (A) Salinity Scan of Kolliphor® PS60 in hexane. (B) concentration dependence of the Cc value for an O/W and W/O emulsifier.
(A) Salinity Scan of Kolliphor® PS60 in hexane. (B) concentration dependence of the Cc value for an O/W and W/O emulsifier. Experimental emulsion droplet radii from the salinity scan shown in Figure 1 compared to predicted values using HLD-NAC Theory
Experimental emulsion droplet radii from the salinity scan shown in Figure 1 compared to predicted values using HLD-NAC Theory Comparison of observed and predicted stability for four representative pharmaceutical emulsions.
Comparison of observed and predicted stability for four representative pharmaceutical emulsions. 