Formulation and Delivery - Biomolecular
(T1230-07-38) Protein Stabilization Sweet Spot: Novel Class of Sugar-Based Excipients Enabling Downstream Processing and Formulation
Tuesday, October 22, 2024
12:30 PM - 1:30 PM MT

Eunice Costa, PhD
R&D Director
Hovione FarmaCiencia SA
Lisboa, Lisboa, Portugal- PL
Paulo Roque Lino, Ph.D.
Senior Scientist
Hovione
Lisboa, Lisboa, Portugal - AF
Ana Filipa Ferreira, Ph.D.
Senior Scientist II / Translational Lead
Hovione
Lisboa, Lisboa, Portugal - EC
Eva C. Lourenço, Ph.D.
Scientist
Hovione
Lisboa, Lisboa, Portugal - JD
Joana Diogo, MSc
Analytical Scientist
Hovione
Lisboa, Lisboa, Portugal - RG
Ricardo Gonçalves, MSc
Senior Analytical Scientist
Hovione
Lisboa, Lisboa, Portugal - OS
Osvaldo S. Ascenso, MSc
Laboratory Technician
Hovione
Lisboa, Lisboa, Portugal - JC
Joana Cristóvão, Ph.D.
Analytical Manager
Hovione
Lisboa, Lisboa, Portugal - LM
Luís Marques, Ph.D.
Senior Scientist
Hovione
Lisboa, Lisboa, Portugal
Presenter (non-author)(s)
Main Author(s)
Co-Author(s)
Co-Author(s)
Purpose: The stabilization of proteins against temperature, acidic pH, and shaking stress is crucial in bioprocessing to maintain therapeutic efficacy and safety. Acidic conditions, common in viral inactivation and chromatography steps, can disrupt protein structure, while temperature and shaking stresses during mixing, transport, and storage can induce denaturation and aggregation. These destabilizing factors hinder the production, purification, and storage of protein therapeutics, leading to the loss of biological activity and potential immune responses. To address these challenges strategies such as using stabilizing excipients (e.g., sugars, polyols, amino acids), and optimizing buffer systems are commonly employed. Here we present the impact of adding novel synthetic sugars to different biomolecules under temperature, shaking, and low pH stress showing that these can provide a significant improvement in the stability of biologics during bioprocessing.
Methods: Shaking stress. mAb A samples (5 mg/mL in 50 mM citrate pH 6.0 buffer) were incubated at RT in a vortex apparatus (1100 rpm) with synthetic sugars at 20 mM. mAb B samples (5 mg/mL in 20 mM PBS pH 7.4 buffer) were incubated at RT in a rocking apparatus with synthetic sugars at 200 mM. Aliquots were analyzed for protein purity using SEC-HPLC. Low pH stress. Aliquots of low pH load material (Humira commercial formulation, 5 mg/mL) were supplemented with synthetic sugars and acidified to target pH 3.2 by adding 1M acetic acid dropwise. Aliquots of the acidified material were neutralized with 1M Tris base to pH 7.5 and analyzed for protein purity using SEC-HPLC. Thermal stress. Aliquots of an enzyme were initially screened by DSF (Activase commercial formulation, 1 mg/mL) and lead conditions followed by an accelerated stability screening at 50ºC throughout 5 days with incubation of the selected leads at 100 mM – monitored by SEC-HPLC.
Results: The stability of mAb A and mAb B to shaking stress conditions was studied under two different protocols (figure 1). mAb A samples were incubated with 7 different novel synthetic sugars at 20 mM and submitted to shaking in a vortex mixer at 1100 RPM at RT (Figure 1, a). The results show that all novel synthetic sugars were able to stabilize mAb A and HS102 was the best stabilizer with 23% delta of monomer content after 60 minutes compared to the control. mAb B was incubated with and without synthetic sugars at 200 mM and submitted to shaking in a rocking mixer at RT (Figure 1, b) and compound HS311 was able to maintain mAb B stability for 96 hours. Low pH stress conditions - pH 3.2 for 12 hours - typically applied during viral inactivation protocols were applied to Humira (commercial formulation of Adalimumab) with and without excipient HS311 spiked in at 500 mM (Figure 2). SEC-HPLC results show an improved stability profile with a delta of monomer content of 14% after 2 hours and 22% after 12 hours in the presence of HS311 when compared to the commercial formulation. Stabilization kinetics monitored by DSF evidenced a significant thermal stabilization potential of every tested sugar within the tested range, of 10-500 mM (Figure 3, a). Lead conditions were selected at 100 mM and throughout an accelerated stability study at 50ºC, a significant reduction of protein aggregation analyzed by SEC was observed throughout 5 days (Figure 3, b).
Conclusion: The promising results observed in the present in-use tests, which mimic acidic virus inactivation steps, mechanical processing and handling stress, and thermal stabilization, indicate that these novel sugar-based excipients could significantly enhance bioprocessing. These improvements suggest that the production of stable, high-quality protein therapeutics could become more reliable and cost-effective, positioning these excipients as pivotal elements in advancing biologics processing and formulation pipelines.
References: Pandey, L. M. (2022). Physicochemical factors of bioprocessing impact the stability of therapeutic proteins. Biotechnology Advances, 55, 107909.
Rahban, M., Ahmad, F., Piatyszek, M. A., Haertlé, T., Saso, L., & Saboury, A. A. (2023). Stabilization challenges and aggregation in protein-based therapeutics in the pharmaceutical industry. RSC Advances, 13(51), 35947-35963.
Wang, W. (1999). Instability, stabilization, and formulation of liquid protein pharmaceuticals. International Journal of Pharmaceutics, 185(2), 129-188.
.jpg) Figure 1. The stabilization and mAb A (5 mg/mL) shaking during 60 minutes in a vortex mixer at 1100 rpm in the presence of synthetic sugars at 20 mM (a) and mAb B (5 mg/mL) during 4 days on a rocking mixer in the presence of synthetic sugars at 200 mM (b) were evaluated by SEC-HPLC – relative monomer content compared to T0.
Figure 1. The stabilization and mAb A (5 mg/mL) shaking during 60 minutes in a vortex mixer at 1100 rpm in the presence of synthetic sugars at 20 mM (a) and mAb B (5 mg/mL) during 4 days on a rocking mixer in the presence of synthetic sugars at 200 mM (b) were evaluated by SEC-HPLC – relative monomer content compared to T0.
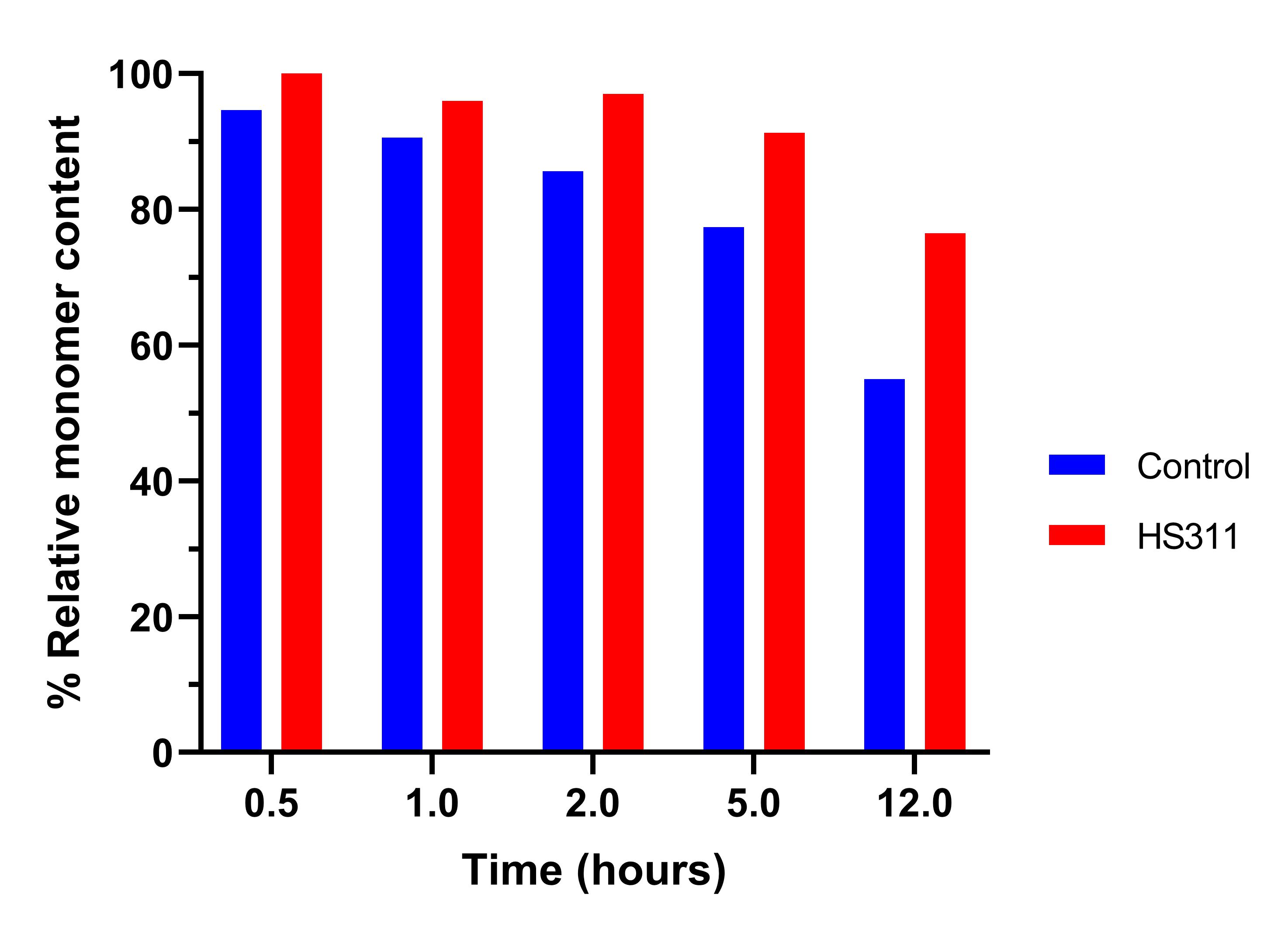 Figure 2. Stabilization of the commercially formulated mAb - Humira® (5 mg/mL) in the presence of synthetic sugar HS311 at 500 mM under low pH stress during 12 hours at pH 3.2 was evaluated by SEC-HPLC – relative monomer content compared to T0.
Figure 2. Stabilization of the commercially formulated mAb - Humira® (5 mg/mL) in the presence of synthetic sugar HS311 at 500 mM under low pH stress during 12 hours at pH 3.2 was evaluated by SEC-HPLC – relative monomer content compared to T0.
.jpg) Differential scanning fluorimetry melting temperature profile of Activase (1mg/mL) at 1ºC/min incubated in the presence of synthetic sugars ranging from 10-500 mM (a) and stress stability profile at 50ºC measuring high molecular weight species by SEC-HPLC evidencing significant protein stabilization of HS at 100 mM throughout 5 days at 50ºC (b).
Differential scanning fluorimetry melting temperature profile of Activase (1mg/mL) at 1ºC/min incubated in the presence of synthetic sugars ranging from 10-500 mM (a) and stress stability profile at 50ºC measuring high molecular weight species by SEC-HPLC evidencing significant protein stabilization of HS at 100 mM throughout 5 days at 50ºC (b).
Methods: Shaking stress. mAb A samples (5 mg/mL in 50 mM citrate pH 6.0 buffer) were incubated at RT in a vortex apparatus (1100 rpm) with synthetic sugars at 20 mM. mAb B samples (5 mg/mL in 20 mM PBS pH 7.4 buffer) were incubated at RT in a rocking apparatus with synthetic sugars at 200 mM. Aliquots were analyzed for protein purity using SEC-HPLC. Low pH stress. Aliquots of low pH load material (Humira commercial formulation, 5 mg/mL) were supplemented with synthetic sugars and acidified to target pH 3.2 by adding 1M acetic acid dropwise. Aliquots of the acidified material were neutralized with 1M Tris base to pH 7.5 and analyzed for protein purity using SEC-HPLC. Thermal stress. Aliquots of an enzyme were initially screened by DSF (Activase commercial formulation, 1 mg/mL) and lead conditions followed by an accelerated stability screening at 50ºC throughout 5 days with incubation of the selected leads at 100 mM – monitored by SEC-HPLC.
Results: The stability of mAb A and mAb B to shaking stress conditions was studied under two different protocols (figure 1). mAb A samples were incubated with 7 different novel synthetic sugars at 20 mM and submitted to shaking in a vortex mixer at 1100 RPM at RT (Figure 1, a). The results show that all novel synthetic sugars were able to stabilize mAb A and HS102 was the best stabilizer with 23% delta of monomer content after 60 minutes compared to the control. mAb B was incubated with and without synthetic sugars at 200 mM and submitted to shaking in a rocking mixer at RT (Figure 1, b) and compound HS311 was able to maintain mAb B stability for 96 hours. Low pH stress conditions - pH 3.2 for 12 hours - typically applied during viral inactivation protocols were applied to Humira (commercial formulation of Adalimumab) with and without excipient HS311 spiked in at 500 mM (Figure 2). SEC-HPLC results show an improved stability profile with a delta of monomer content of 14% after 2 hours and 22% after 12 hours in the presence of HS311 when compared to the commercial formulation. Stabilization kinetics monitored by DSF evidenced a significant thermal stabilization potential of every tested sugar within the tested range, of 10-500 mM (Figure 3, a). Lead conditions were selected at 100 mM and throughout an accelerated stability study at 50ºC, a significant reduction of protein aggregation analyzed by SEC was observed throughout 5 days (Figure 3, b).
Conclusion: The promising results observed in the present in-use tests, which mimic acidic virus inactivation steps, mechanical processing and handling stress, and thermal stabilization, indicate that these novel sugar-based excipients could significantly enhance bioprocessing. These improvements suggest that the production of stable, high-quality protein therapeutics could become more reliable and cost-effective, positioning these excipients as pivotal elements in advancing biologics processing and formulation pipelines.
References: Pandey, L. M. (2022). Physicochemical factors of bioprocessing impact the stability of therapeutic proteins. Biotechnology Advances, 55, 107909.
Rahban, M., Ahmad, F., Piatyszek, M. A., Haertlé, T., Saso, L., & Saboury, A. A. (2023). Stabilization challenges and aggregation in protein-based therapeutics in the pharmaceutical industry. RSC Advances, 13(51), 35947-35963.
Wang, W. (1999). Instability, stabilization, and formulation of liquid protein pharmaceuticals. International Journal of Pharmaceutics, 185(2), 129-188.
.jpg) Figure 1. The stabilization and mAb A (5 mg/mL) shaking during 60 minutes in a vortex mixer at 1100 rpm in the presence of synthetic sugars at 20 mM (a) and mAb B (5 mg/mL) during 4 days on a rocking mixer in the presence of synthetic sugars at 200 mM (b) were evaluated by SEC-HPLC – relative monomer content compared to T0.
Figure 1. The stabilization and mAb A (5 mg/mL) shaking during 60 minutes in a vortex mixer at 1100 rpm in the presence of synthetic sugars at 20 mM (a) and mAb B (5 mg/mL) during 4 days on a rocking mixer in the presence of synthetic sugars at 200 mM (b) were evaluated by SEC-HPLC – relative monomer content compared to T0. Figure 2. Stabilization of the commercially formulated mAb - Humira® (5 mg/mL) in the presence of synthetic sugar HS311 at 500 mM under low pH stress during 12 hours at pH 3.2 was evaluated by SEC-HPLC – relative monomer content compared to T0.
Figure 2. Stabilization of the commercially formulated mAb - Humira® (5 mg/mL) in the presence of synthetic sugar HS311 at 500 mM under low pH stress during 12 hours at pH 3.2 was evaluated by SEC-HPLC – relative monomer content compared to T0..jpg) Differential scanning fluorimetry melting temperature profile of Activase (1mg/mL) at 1ºC/min incubated in the presence of synthetic sugars ranging from 10-500 mM (a) and stress stability profile at 50ºC measuring high molecular weight species by SEC-HPLC evidencing significant protein stabilization of HS at 100 mM throughout 5 days at 50ºC (b).
Differential scanning fluorimetry melting temperature profile of Activase (1mg/mL) at 1ºC/min incubated in the presence of synthetic sugars ranging from 10-500 mM (a) and stress stability profile at 50ºC measuring high molecular weight species by SEC-HPLC evidencing significant protein stabilization of HS at 100 mM throughout 5 days at 50ºC (b).