Preclinical, Clinical, and Translational Sciences
(M0930-10-54) Utilization of Proteomic Surrogates for Early Detection of Unexpected Drug Benefits
Monday, October 21, 2024
9:30 AM - 10:30 AM MT

Jessica Chadwick, PhD
Senior Scientist
SomaLogic
Boulder, Colorado, United States
Jessica Chadwick, PhD
Senior Scientist
SomaLogic
Boulder, Colorado, United States- MH
Michael Hinterberg, Ph.D.
Senior Scientist
SomaLogic
Boulder, Colorado, United States - CP
Clare Paterson, Ph.D.
Director
SomaLogic
Boulder, Colorado, United States - SS
Sama Shrestha, Ph.D.
Scientist I
SomaLogic
Boulder, Colorado, United States - ET
Emma Troth, Ph.D.
Scientist I
SomaLogic
Boulder, Colorado, United States - SW
Steve Williams, M.D.
Chief Medical Officer
SomaLogic
Boulder, Colorado, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Detection of benefits and adverse effects of therapies in earlier clinical trial phases could improve the safety, efficiency, and cost of clinical outcome trials. For example, while sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP-1 RA) are recognized success stories, their extensive and unexpected benefits manifested late in development. Earlier identification of their benefits beyond improved glycemic control could have enabled planning for new outcomes studies to take place much earlier and more efficiently and improved patient outcomes. Using the Exenatide Study of Cardiovascular Event Lowering (EXSCEL) and Studies of Empagliflozin and Its Cardiovascular, Renal and Metabolic Effects in Patients with Diabetes Mellitus, or Prediabetes, and Heart Failure (SUGAR-DM-HF) trials as examples to study GLP1-RA and SGLT2i responses, we hypothesized that previously validated proteomic surrogates of cardiovascular (CV) events1, kidney health2 and key cardiometabolic measures2,3 could have enabled detection of these unexpected drug benefits earlier in development in a smaller number of participants over a shorter follow-up period.
Methods: The EXSCEL trial randomized 14,752 type 2 diabetes patients to once weekly exenatide versus placebo4. SomaScan® was run in a biomarker substudy of 5205 participants on baseline and 1-year plasma samples. The SUGAR-DM-HF trial randomized 105 patients to empagliflozin once daily or placebo, stratified by age ( < 65 and ≥65 years) and glycemic status (DM or Prediabetes)5. SomaScan® was run on all participants on baseline, 3-month, and 9-month plasma samples. SomaSignal® tests (each derived from ~5000 plasma proteins measurements using SomaScan assay6,7) were applied to paired plasma samples at baseline and 9-months (SUGAR-DM-HF) or 1-year (EXSCEL) in intervention (EXSCEL n=1840; SUGAR-DM-HF n=45) and control (EXSCEL n=1833; SUGAR-DM-HF n=52) participants. SomaSignal tests with binary class outputs (glucose tolerance, liver fat, kidney health) used proportional tests to determine if the proportions predicted to change in control and respective intervention groups were significantly different. SomaSignal tests with continuous outputs (CV risk, heart failure prognosis, cardiorespiratory fitness, lean body mass, percent body fat, visceral fat, and resting energy rate) used paired t-tests to evaluate changes in predictions. Resulting p-values were assessed for significance (alpha=0.05) after Bonferroni adjustment for the independent SomaSignal tests. Power calculations were performed using EXSCEL effect size distributions to determine the minimum number of samples needed to detect a significant change within the treatment period (alpha = 0.05; 80% power) using a t-test comparing two-sample means.
Results: Overall changes in SomaSignal test results were consistent with published outcome studies and known effects of these drug classes. We demonstrated that attenuation of CV risk and improvement of cardiometabolic traits with exenatide (see Figure 1) was detectable within 1-year (p=0.002) in sample sizes significantly smaller than the original outcomes study (n=1368 vs. n >7000). Similarly, cardioprotective (p=0.06) and renal protective (p=0.037) effects of empagliflozin (see Figure 2) were detectable within 36 weeks using SomaSignal tests in small sample sizes (n ~ 50) compared to published outcomes studies requiring thousands of participants followed for >2 years. Power calculations run on the EXSCEL study revealed sample sizes could be further reduced and still detect significant differences. Significant changes in cardiovascular risk could be seen with 1368 samples per group, while some of the physical fitness measures like lean body mass and resting energy rate could detect significant differences with only a few hundred samples.
Conclusion: SomaSignal tests were able to predict cardiometabolic benefits of GLP-1 RA and SGLT2i drugs providing evidence that proteomics may provide a powerful tool for decreasing the burden to clinical trial participants, optimizing pipelines of drug development and better targeting outcome trials, by predicting effects of novel therapeutics in smaller, shorter studies.
References: 1. Williams SA, Ostroff R, Hinterberg MA, Coresh J, Ballantyne CM, Matsushita K, et al. A proteomic surrogate for cardiovascular outcomes that is sensitive to multiple mechanisms of change in risk. Sci Transl Med. 2022;14:eabj9625.
2. Dark HE, Paterson C, Daya GN, Peng Z, Duggan MR, Bilgel M, et al. Proteomic Indicators of Health Predict Alzheimer's Disease Biomarker Levels and Dementia Risk. Ann Neurol. 2024;95(2):260-273.
3. Williams SA, Kivimaki M, Langenberg C, Hingorani AD, Casas JP, Bouchard C, et al. Plasma protein patterns as comprehensive indicators of health. Nat Med. 2019;25:1851-1857.
4. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. The New England Journal of Medicine 2017;377:1228-1239.
5. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients with Type 2 Diabetes, or Prediabetes, and Heart Failure with Reduced Ejection Fraction (SUGAR-DM-HF). Circulation. 2021 9;143(6):516-525.
6. Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoSOne 2010;5:e15004.
7. Rohloff JC, Gelinas AD, Jarvis TC, Ochsner UA, Schneider DJ, Gold L, Janjic N. Nucleic Acid Ligands with protein-like Side Chains: Modified Aptamers and Their Use as Diagnostic and Therapeutic Agents. Molecular Therapy Nucleic Acids 2014;3:e201
.jpg) Figure 1: Relative percent change in cardiometabolic traits (measured by SomaSignal tests) by treatment arm (N=1787 placebo arm; N=1812 exenatide arm) from baseline to 12 months in the EXSCEL trial. Changes marked with an asterisk (*) are a statistically significant change at alpha=0.05. NOTE: Alcohol Impact was included as a negative control and was not expected to change with treatment.
Figure 1: Relative percent change in cardiometabolic traits (measured by SomaSignal tests) by treatment arm (N=1787 placebo arm; N=1812 exenatide arm) from baseline to 12 months in the EXSCEL trial. Changes marked with an asterisk (*) are a statistically significant change at alpha=0.05. NOTE: Alcohol Impact was included as a negative control and was not expected to change with treatment.
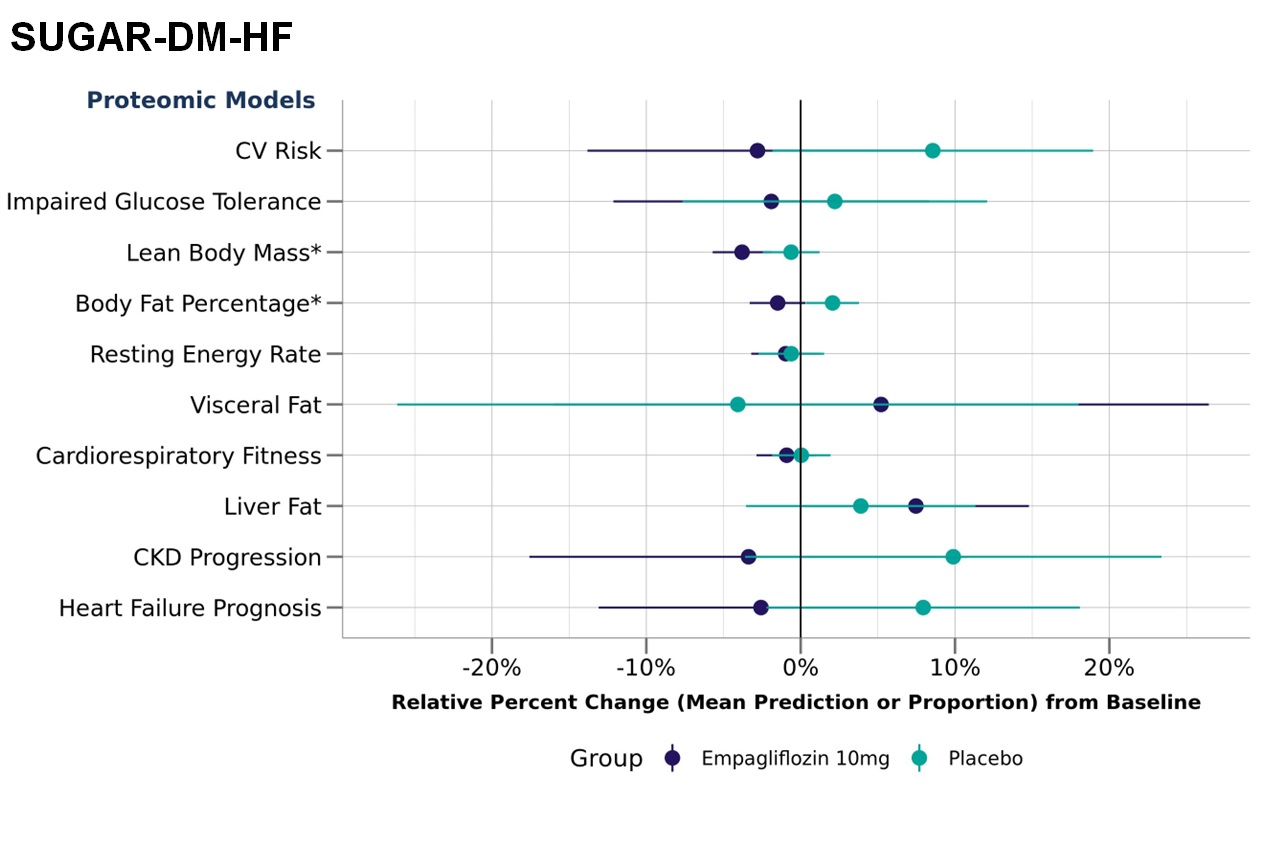 Figure 2: Relative percent change in cardiometabolic traits (measured by SomaSignal tests) by treatment arm (N= 53 placebo arm; N=47 empagliflozin arm) from baseline to 36 weeks in the SUGAR-DM-HF trial. Changes marked with an asterisk (*) are a statistically significant change at alpha=0.05.
Figure 2: Relative percent change in cardiometabolic traits (measured by SomaSignal tests) by treatment arm (N= 53 placebo arm; N=47 empagliflozin arm) from baseline to 36 weeks in the SUGAR-DM-HF trial. Changes marked with an asterisk (*) are a statistically significant change at alpha=0.05.
Methods: The EXSCEL trial randomized 14,752 type 2 diabetes patients to once weekly exenatide versus placebo4. SomaScan® was run in a biomarker substudy of 5205 participants on baseline and 1-year plasma samples. The SUGAR-DM-HF trial randomized 105 patients to empagliflozin once daily or placebo, stratified by age ( < 65 and ≥65 years) and glycemic status (DM or Prediabetes)5. SomaScan® was run on all participants on baseline, 3-month, and 9-month plasma samples. SomaSignal® tests (each derived from ~5000 plasma proteins measurements using SomaScan assay6,7) were applied to paired plasma samples at baseline and 9-months (SUGAR-DM-HF) or 1-year (EXSCEL) in intervention (EXSCEL n=1840; SUGAR-DM-HF n=45) and control (EXSCEL n=1833; SUGAR-DM-HF n=52) participants. SomaSignal tests with binary class outputs (glucose tolerance, liver fat, kidney health) used proportional tests to determine if the proportions predicted to change in control and respective intervention groups were significantly different. SomaSignal tests with continuous outputs (CV risk, heart failure prognosis, cardiorespiratory fitness, lean body mass, percent body fat, visceral fat, and resting energy rate) used paired t-tests to evaluate changes in predictions. Resulting p-values were assessed for significance (alpha=0.05) after Bonferroni adjustment for the independent SomaSignal tests. Power calculations were performed using EXSCEL effect size distributions to determine the minimum number of samples needed to detect a significant change within the treatment period (alpha = 0.05; 80% power) using a t-test comparing two-sample means.
Results: Overall changes in SomaSignal test results were consistent with published outcome studies and known effects of these drug classes. We demonstrated that attenuation of CV risk and improvement of cardiometabolic traits with exenatide (see Figure 1) was detectable within 1-year (p=0.002) in sample sizes significantly smaller than the original outcomes study (n=1368 vs. n >7000). Similarly, cardioprotective (p=0.06) and renal protective (p=0.037) effects of empagliflozin (see Figure 2) were detectable within 36 weeks using SomaSignal tests in small sample sizes (n ~ 50) compared to published outcomes studies requiring thousands of participants followed for >2 years. Power calculations run on the EXSCEL study revealed sample sizes could be further reduced and still detect significant differences. Significant changes in cardiovascular risk could be seen with 1368 samples per group, while some of the physical fitness measures like lean body mass and resting energy rate could detect significant differences with only a few hundred samples.
Conclusion: SomaSignal tests were able to predict cardiometabolic benefits of GLP-1 RA and SGLT2i drugs providing evidence that proteomics may provide a powerful tool for decreasing the burden to clinical trial participants, optimizing pipelines of drug development and better targeting outcome trials, by predicting effects of novel therapeutics in smaller, shorter studies.
References: 1. Williams SA, Ostroff R, Hinterberg MA, Coresh J, Ballantyne CM, Matsushita K, et al. A proteomic surrogate for cardiovascular outcomes that is sensitive to multiple mechanisms of change in risk. Sci Transl Med. 2022;14:eabj9625.
2. Dark HE, Paterson C, Daya GN, Peng Z, Duggan MR, Bilgel M, et al. Proteomic Indicators of Health Predict Alzheimer's Disease Biomarker Levels and Dementia Risk. Ann Neurol. 2024;95(2):260-273.
3. Williams SA, Kivimaki M, Langenberg C, Hingorani AD, Casas JP, Bouchard C, et al. Plasma protein patterns as comprehensive indicators of health. Nat Med. 2019;25:1851-1857.
4. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. The New England Journal of Medicine 2017;377:1228-1239.
5. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients with Type 2 Diabetes, or Prediabetes, and Heart Failure with Reduced Ejection Fraction (SUGAR-DM-HF). Circulation. 2021 9;143(6):516-525.
6. Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoSOne 2010;5:e15004.
7. Rohloff JC, Gelinas AD, Jarvis TC, Ochsner UA, Schneider DJ, Gold L, Janjic N. Nucleic Acid Ligands with protein-like Side Chains: Modified Aptamers and Their Use as Diagnostic and Therapeutic Agents. Molecular Therapy Nucleic Acids 2014;3:e201
.jpg) Figure 1: Relative percent change in cardiometabolic traits (measured by SomaSignal tests) by treatment arm (N=1787 placebo arm; N=1812 exenatide arm) from baseline to 12 months in the EXSCEL trial. Changes marked with an asterisk (*) are a statistically significant change at alpha=0.05. NOTE: Alcohol Impact was included as a negative control and was not expected to change with treatment.
Figure 1: Relative percent change in cardiometabolic traits (measured by SomaSignal tests) by treatment arm (N=1787 placebo arm; N=1812 exenatide arm) from baseline to 12 months in the EXSCEL trial. Changes marked with an asterisk (*) are a statistically significant change at alpha=0.05. NOTE: Alcohol Impact was included as a negative control and was not expected to change with treatment. Figure 2: Relative percent change in cardiometabolic traits (measured by SomaSignal tests) by treatment arm (N= 53 placebo arm; N=47 empagliflozin arm) from baseline to 36 weeks in the SUGAR-DM-HF trial. Changes marked with an asterisk (*) are a statistically significant change at alpha=0.05.
Figure 2: Relative percent change in cardiometabolic traits (measured by SomaSignal tests) by treatment arm (N= 53 placebo arm; N=47 empagliflozin arm) from baseline to 36 weeks in the SUGAR-DM-HF trial. Changes marked with an asterisk (*) are a statistically significant change at alpha=0.05.