Formulation and Delivery - Biomolecular
(M1030-07-37) GRP78 Targeting of Long-Acting Hydra-Elastin-Like Polypeptides (ELPs) Carrying Rapamycin to Breast Cancer
Monday, October 21, 2024
10:30 AM - 11:30 AM MT
- SA
Sara Aly Attia, BS
Ph.D. Candidate
University of Southern California
Los Angeles, California, United States - JM
J. Andrew MacKay, Ph.D.
Professor
University of Southern California
N/A, California, United States
Presenting Author(s)
Main Author(s)
Purpose: Rapamycin (Rapa) and related Everolimus have been recognized and approved, respectively, to slow breast cancer progression; however, their use is limited due to solubility, side effects, and resistance . By forming a complex with the FK506-Binding Protein (FKPB), they directly inhibit mTORC-1. This reduces activation of S6 kinase 1 (S6K1) and phosphorylation of ribosomal protein S6 (rpS6) [3]. P-rpS6 protein levels activate 5' terminal oligopyrimidine (5'TOP) mRNA, which modulates translational machinery relevant to cancer [4]. Since GRP78 is a common upregulated hallmark of cancer, we engineered GRP78 targeting ligand for selective Rapa delivery [5]. Recently we conjugated a panel of GRP78-targeting ligands to a soluble, multi-headed ‘hydra’ carrier composed of 5 FKBP binding domains spaced between the elastin-like polypeptide (ELP) of the sequence (VPGAG)24. Based on their cellular association and activity in BT474 cells, we concluded the L-peptide (RLLDTNRPLLPY) best promotes activity of Rapa [6]. Herein, we now report a long-acting ‘hydra ELP’ called L-5FV (Figure 1). This consists of the L-peptide and five Rapa binding domains (FKPB), linked by 4 ELPs of the sequence (VPGVG)24. L-peptide distinctly targets cellular GRP78, while FKPB improves Rapa solubility and cellular uptake. Unlike the soluble L-5FA carrier, this L-5FV carrier undergoes ELP-mediated phase separation at body temperature, which extends their absorption in-vivo [7, 8].
Methods: Molecular cloning was confirmed by Sanger sequencing and protein purity and identity was verified by SDS-PAGE and MALDI-TOF. ELP assembly was characterized by UV−vis spectrophotometry (Figure 2A) and light scattering (SEC-MALS, DLS). Binding affinity between FKPB and Rapa was studied by Surface Plasmon Resonance (SPR) (Figure 2B). Cellular uptake (confocal microscopy, anti-ELP WB) (Figure 3i, 3ii) and activity (Figure 3iii) (rpS6 phosphorylation, and viability WST formazan assay) were compared between free Rapa, 5FV/Rapa, and L-5FV/Rapa on the hormone receptor positive BT474 breast cancer cell line.
Results: Physiochemical characterization results confirmed successful expression (25 mg/L) of the targeted thermo-responsive depot. Rapa encapsulation moderately increased the ELP transition temperature (Tt), which were 28, 22.5, 33.7, and 33.4 ºC for 5FV, L-5FV, 5FV-Rapa, and L-5FV-Rapa, respectively at 25 µM. Their association with Rapa has a nanomolar binding affinity (0.8 nM for 5FV and 0.4 nM for L-5FV). Compared to control 5FV, the GRP78 L-peptide promoted 2-fold more cellular association (***P = 0.0006) and 2-fold more potent inhibition of mTORC1 signaling (*P = 0.04). Data were replicated independently at different cell passage timing and analyzed by One-Way Analysis of Variance ‘ANOVA’, followed by the Tukey post-hoc test.
Conclusion: L-5FV has a strong binding affinity to Rapa, thermosensitive behavior at physiological temperature, and selective GRP78 targeting that prompts a cellular response to Rapa through inhibition of P-rpS6 signaling. This is a commonly accepted readout for mTORC-1 activity relevant to invasive breast ductal carcinoma. Future studies will compare the relative potency of these targeted and untargeted depot systems in-vivo.
References: 1. Simamora, P., J.M. Alvarez, and S.H. Yalkowsky, Solubilization of rapamycin. International Journal of Pharmaceutics, 2001. 213(1): p. 25-29.
2. Baselga, J., et al., Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med, 2012. 366(6): p. 520-9.
3. Zhou, H. and S. Huang, The complexes of mammalian target of rapamycin. Curr Protein Pept Sci, 2010. 11(6): p. 409-24.
4. Nandagopal, N. and P.P. Roux, Regulation of global and specific mRNA translation by the mTOR signaling pathway. Translation (Austin), 2015. 3(1): p. e983402.
5. Wang, M., et al., Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal, 2009. 11(9): p. 2307-16.
6. Avila, H., et al., Hydra-Elastin-like Polypeptides Increase Rapamycin Potency When Targeting Cell Surface GRP78. Biomacromolecules, 2022. 23(8): p. 3116-3129.
7. Roberts, S., M. Dzuricky, and A. Chilkoti, Elastin-like polypeptides as models of intrinsically disordered proteins. FEBS Lett, 2015. 589(19 Pt A): p. 2477-86.
8. Ju, Y., et al., Intralacrimal Sustained Delivery of Rapamycin Shows Therapeutic Effects without Systemic Toxicity in a Mouse Model of Autoimmune Dacryoadenitis Characteristic of Sjögren's Syndrome. Biomacromolecules, 2021. 22(3): p. 1102-1114.
Acknowledgements: This work was made possible by University of Southern California (USC), the G.S.H. Professorship, National Institutes of Health R01 GM114839 to JAM, R01EY026635 to JAM, R01-CA027607 to ASL, P30 CA014089 to the USC Norris Comprehensive Cancer Center (USC NCCC), and P30 EY029220 to the USC Ophthalmology Center Core Grant for Vision Research. A Programmatic Pilot grant from the USC NCCC Translational and Clinical Sciences Program funded part of this work. Thank you to Alan Epstein and Cancer Therapeutics Laboratories, Inc. for providing anti-ELP AK-1 antibodies.
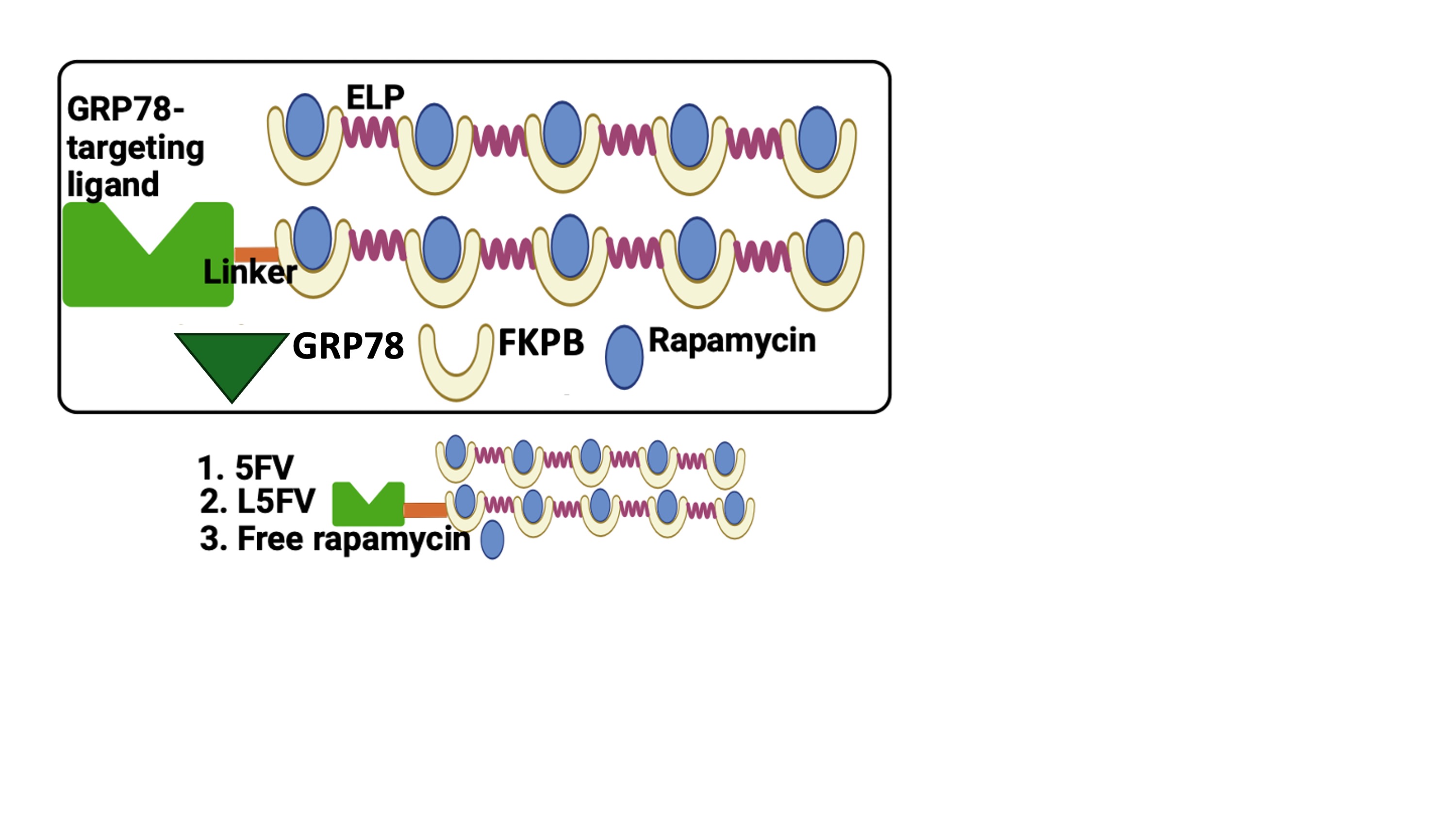 Figure 1. GRP78-targeting FKPB-ELP fusions for treatment groups employed in the study (GRP78-targeted L-5FV, Untargeted 5FV, Free Rapa).
Figure 1. GRP78-targeting FKPB-ELP fusions for treatment groups employed in the study (GRP78-targeted L-5FV, Untargeted 5FV, Free Rapa).
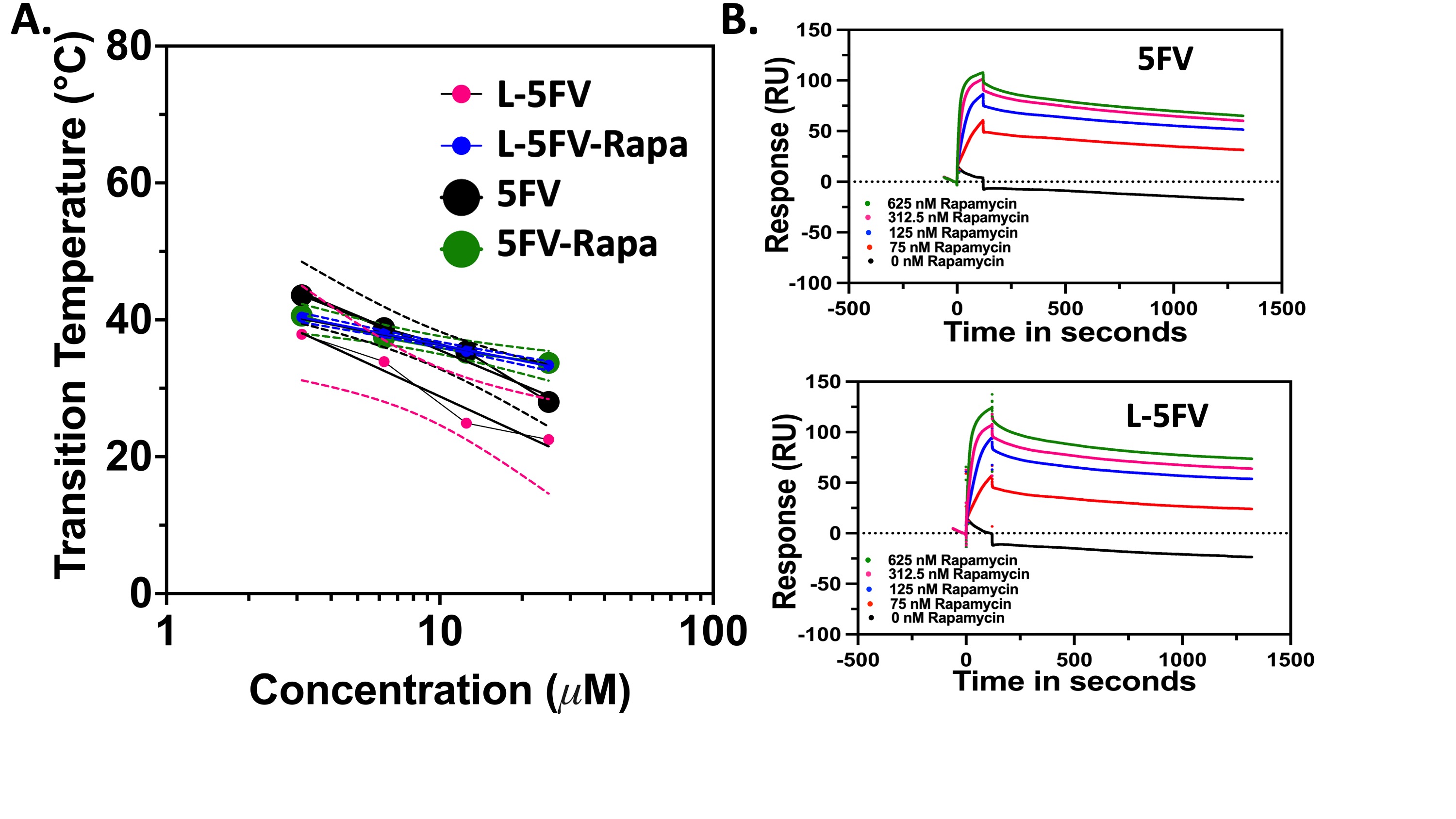 Figure 2. Rapa binds to FKBP-ELPs with and without the GRP78 targeting L-peptide. Rapa binding slightly raises the phase transition temperature of 5FV and L-5FV fusions. A) Optical-density was used to measure Tt for purified 5FV, L-5FV, 5FV-Rapa, and L-5FV-Rapa formulations (15-80 ºC) at a wavelength of 350 nm over a range of concentrations (25, 12.5, 6.25, and 3.13 µM) in PBS. The maximum first derivative was defined as Tt. As shown, Tt was log-linear with the concentration. B) SPR quantified the affinity of 5FV and L-5FV to Rapa over a range of concentrations (625, 312.5, 125, and 75 nM) below Tt. Rapa bound to 5FV and L-5FV with less than 1 nM equilibrium binding constant.
Figure 2. Rapa binds to FKBP-ELPs with and without the GRP78 targeting L-peptide. Rapa binding slightly raises the phase transition temperature of 5FV and L-5FV fusions. A) Optical-density was used to measure Tt for purified 5FV, L-5FV, 5FV-Rapa, and L-5FV-Rapa formulations (15-80 ºC) at a wavelength of 350 nm over a range of concentrations (25, 12.5, 6.25, and 3.13 µM) in PBS. The maximum first derivative was defined as Tt. As shown, Tt was log-linear with the concentration. B) SPR quantified the affinity of 5FV and L-5FV to Rapa over a range of concentrations (625, 312.5, 125, and 75 nM) below Tt. Rapa bound to 5FV and L-5FV with less than 1 nM equilibrium binding constant.
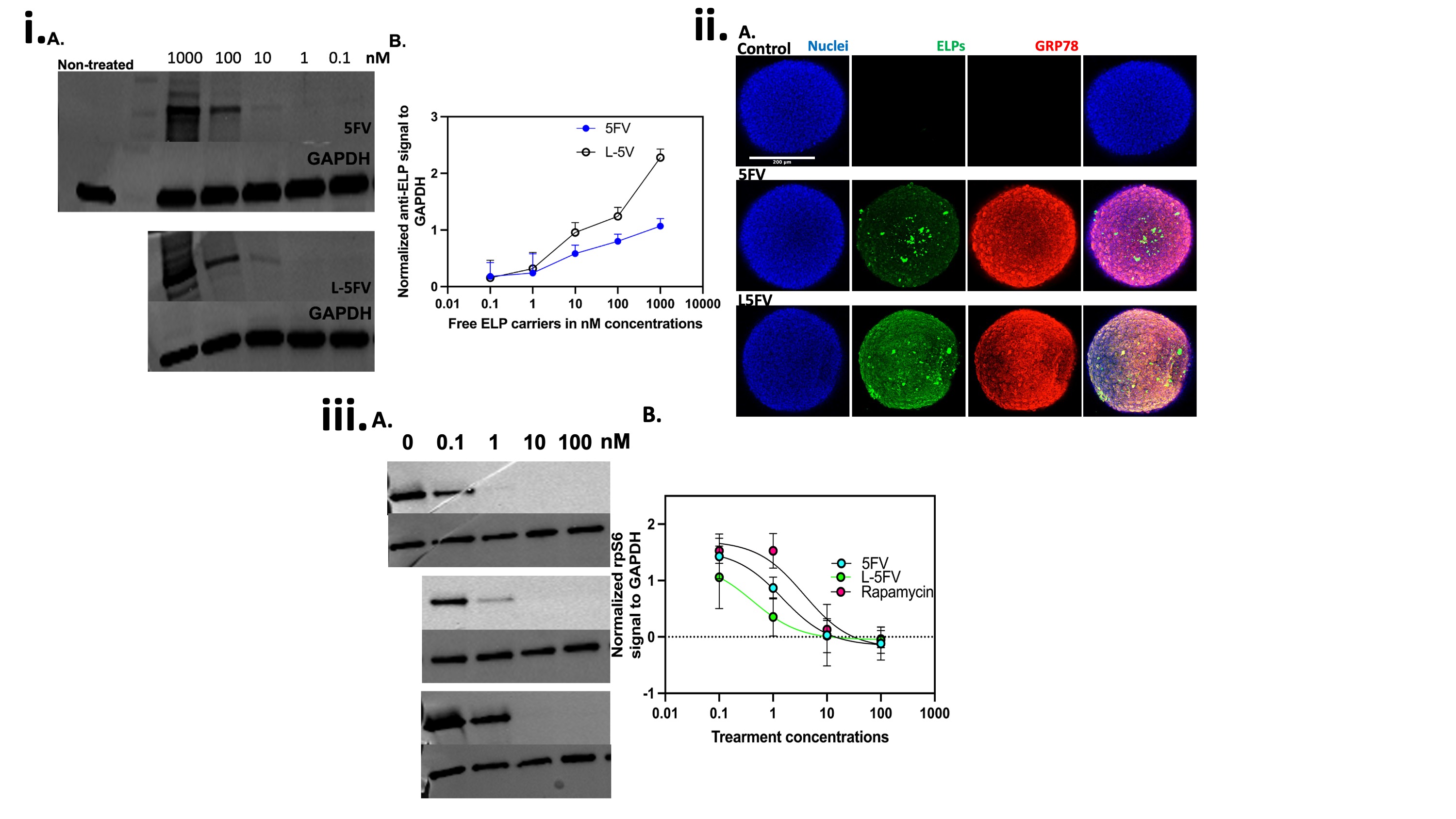 Figure 3. L-5FV/Rapa undergoes more dose-dependent cell association and inhibition of P-rpS6 than 5FV. Rapa inhibits cancer proliferation through inhibition of mTORC-1 in BT474 cells. Compared to 5FV, i) Western blotting against ELP using an anti-ELP antibody and ii) confocal microscopy of 3D spheroids stained using an anti-ELP antibody revealed more L-5FV cellular association at 10 to 1000 nM concentrations. L-5FV/Rapa had significant cellular internalization at the spheroid surface as well as the interior compared to 5FV/Rapa. iii) Demonstrating their relative delivery of Rapa, BT474 cells were incubated with 100, 10, 1, and 0.1 nM Rapa bound to 5FV, L-5FV or free Rapa. P-rpS6 signal by WB was normalized to GAPDH, which revealed the most potent suppression mTORC-1 for GRP-78 targeted L-5FV (**P = 0.001 AND *P = 0.04), relative to 5FV-Rapa AND free Rapa groups, respectively.
Figure 3. L-5FV/Rapa undergoes more dose-dependent cell association and inhibition of P-rpS6 than 5FV. Rapa inhibits cancer proliferation through inhibition of mTORC-1 in BT474 cells. Compared to 5FV, i) Western blotting against ELP using an anti-ELP antibody and ii) confocal microscopy of 3D spheroids stained using an anti-ELP antibody revealed more L-5FV cellular association at 10 to 1000 nM concentrations. L-5FV/Rapa had significant cellular internalization at the spheroid surface as well as the interior compared to 5FV/Rapa. iii) Demonstrating their relative delivery of Rapa, BT474 cells were incubated with 100, 10, 1, and 0.1 nM Rapa bound to 5FV, L-5FV or free Rapa. P-rpS6 signal by WB was normalized to GAPDH, which revealed the most potent suppression mTORC-1 for GRP-78 targeted L-5FV (**P = 0.001 AND *P = 0.04), relative to 5FV-Rapa AND free Rapa groups, respectively.
Methods: Molecular cloning was confirmed by Sanger sequencing and protein purity and identity was verified by SDS-PAGE and MALDI-TOF. ELP assembly was characterized by UV−vis spectrophotometry (Figure 2A) and light scattering (SEC-MALS, DLS). Binding affinity between FKPB and Rapa was studied by Surface Plasmon Resonance (SPR) (Figure 2B). Cellular uptake (confocal microscopy, anti-ELP WB) (Figure 3i, 3ii) and activity (Figure 3iii) (rpS6 phosphorylation, and viability WST formazan assay) were compared between free Rapa, 5FV/Rapa, and L-5FV/Rapa on the hormone receptor positive BT474 breast cancer cell line.
Results: Physiochemical characterization results confirmed successful expression (25 mg/L) of the targeted thermo-responsive depot. Rapa encapsulation moderately increased the ELP transition temperature (Tt), which were 28, 22.5, 33.7, and 33.4 ºC for 5FV, L-5FV, 5FV-Rapa, and L-5FV-Rapa, respectively at 25 µM. Their association with Rapa has a nanomolar binding affinity (0.8 nM for 5FV and 0.4 nM for L-5FV). Compared to control 5FV, the GRP78 L-peptide promoted 2-fold more cellular association (***P = 0.0006) and 2-fold more potent inhibition of mTORC1 signaling (*P = 0.04). Data were replicated independently at different cell passage timing and analyzed by One-Way Analysis of Variance ‘ANOVA’, followed by the Tukey post-hoc test.
Conclusion: L-5FV has a strong binding affinity to Rapa, thermosensitive behavior at physiological temperature, and selective GRP78 targeting that prompts a cellular response to Rapa through inhibition of P-rpS6 signaling. This is a commonly accepted readout for mTORC-1 activity relevant to invasive breast ductal carcinoma. Future studies will compare the relative potency of these targeted and untargeted depot systems in-vivo.
References: 1. Simamora, P., J.M. Alvarez, and S.H. Yalkowsky, Solubilization of rapamycin. International Journal of Pharmaceutics, 2001. 213(1): p. 25-29.
2. Baselga, J., et al., Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med, 2012. 366(6): p. 520-9.
3. Zhou, H. and S. Huang, The complexes of mammalian target of rapamycin. Curr Protein Pept Sci, 2010. 11(6): p. 409-24.
4. Nandagopal, N. and P.P. Roux, Regulation of global and specific mRNA translation by the mTOR signaling pathway. Translation (Austin), 2015. 3(1): p. e983402.
5. Wang, M., et al., Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal, 2009. 11(9): p. 2307-16.
6. Avila, H., et al., Hydra-Elastin-like Polypeptides Increase Rapamycin Potency When Targeting Cell Surface GRP78. Biomacromolecules, 2022. 23(8): p. 3116-3129.
7. Roberts, S., M. Dzuricky, and A. Chilkoti, Elastin-like polypeptides as models of intrinsically disordered proteins. FEBS Lett, 2015. 589(19 Pt A): p. 2477-86.
8. Ju, Y., et al., Intralacrimal Sustained Delivery of Rapamycin Shows Therapeutic Effects without Systemic Toxicity in a Mouse Model of Autoimmune Dacryoadenitis Characteristic of Sjögren's Syndrome. Biomacromolecules, 2021. 22(3): p. 1102-1114.
Acknowledgements: This work was made possible by University of Southern California (USC), the G.S.H. Professorship, National Institutes of Health R01 GM114839 to JAM, R01EY026635 to JAM, R01-CA027607 to ASL, P30 CA014089 to the USC Norris Comprehensive Cancer Center (USC NCCC), and P30 EY029220 to the USC Ophthalmology Center Core Grant for Vision Research. A Programmatic Pilot grant from the USC NCCC Translational and Clinical Sciences Program funded part of this work. Thank you to Alan Epstein and Cancer Therapeutics Laboratories, Inc. for providing anti-ELP AK-1 antibodies.
 Figure 1. GRP78-targeting FKPB-ELP fusions for treatment groups employed in the study (GRP78-targeted L-5FV, Untargeted 5FV, Free Rapa).
Figure 1. GRP78-targeting FKPB-ELP fusions for treatment groups employed in the study (GRP78-targeted L-5FV, Untargeted 5FV, Free Rapa).  Figure 2. Rapa binds to FKBP-ELPs with and without the GRP78 targeting L-peptide. Rapa binding slightly raises the phase transition temperature of 5FV and L-5FV fusions. A) Optical-density was used to measure Tt for purified 5FV, L-5FV, 5FV-Rapa, and L-5FV-Rapa formulations (15-80 ºC) at a wavelength of 350 nm over a range of concentrations (25, 12.5, 6.25, and 3.13 µM) in PBS. The maximum first derivative was defined as Tt. As shown, Tt was log-linear with the concentration. B) SPR quantified the affinity of 5FV and L-5FV to Rapa over a range of concentrations (625, 312.5, 125, and 75 nM) below Tt. Rapa bound to 5FV and L-5FV with less than 1 nM equilibrium binding constant.
Figure 2. Rapa binds to FKBP-ELPs with and without the GRP78 targeting L-peptide. Rapa binding slightly raises the phase transition temperature of 5FV and L-5FV fusions. A) Optical-density was used to measure Tt for purified 5FV, L-5FV, 5FV-Rapa, and L-5FV-Rapa formulations (15-80 ºC) at a wavelength of 350 nm over a range of concentrations (25, 12.5, 6.25, and 3.13 µM) in PBS. The maximum first derivative was defined as Tt. As shown, Tt was log-linear with the concentration. B) SPR quantified the affinity of 5FV and L-5FV to Rapa over a range of concentrations (625, 312.5, 125, and 75 nM) below Tt. Rapa bound to 5FV and L-5FV with less than 1 nM equilibrium binding constant. Figure 3. L-5FV/Rapa undergoes more dose-dependent cell association and inhibition of P-rpS6 than 5FV. Rapa inhibits cancer proliferation through inhibition of mTORC-1 in BT474 cells. Compared to 5FV, i) Western blotting against ELP using an anti-ELP antibody and ii) confocal microscopy of 3D spheroids stained using an anti-ELP antibody revealed more L-5FV cellular association at 10 to 1000 nM concentrations. L-5FV/Rapa had significant cellular internalization at the spheroid surface as well as the interior compared to 5FV/Rapa. iii) Demonstrating their relative delivery of Rapa, BT474 cells were incubated with 100, 10, 1, and 0.1 nM Rapa bound to 5FV, L-5FV or free Rapa. P-rpS6 signal by WB was normalized to GAPDH, which revealed the most potent suppression mTORC-1 for GRP-78 targeted L-5FV (**P = 0.001 AND *P = 0.04), relative to 5FV-Rapa AND free Rapa groups, respectively.
Figure 3. L-5FV/Rapa undergoes more dose-dependent cell association and inhibition of P-rpS6 than 5FV. Rapa inhibits cancer proliferation through inhibition of mTORC-1 in BT474 cells. Compared to 5FV, i) Western blotting against ELP using an anti-ELP antibody and ii) confocal microscopy of 3D spheroids stained using an anti-ELP antibody revealed more L-5FV cellular association at 10 to 1000 nM concentrations. L-5FV/Rapa had significant cellular internalization at the spheroid surface as well as the interior compared to 5FV/Rapa. iii) Demonstrating their relative delivery of Rapa, BT474 cells were incubated with 100, 10, 1, and 0.1 nM Rapa bound to 5FV, L-5FV or free Rapa. P-rpS6 signal by WB was normalized to GAPDH, which revealed the most potent suppression mTORC-1 for GRP-78 targeted L-5FV (**P = 0.001 AND *P = 0.04), relative to 5FV-Rapa AND free Rapa groups, respectively. 