Manufacturing and Analytical Characterization - Chemical
(M1330-10-55) Evaluation of Critical Microstructural and Performance Attributes of Azelaic Acid Topical Gels

Ahmed S. Zidan, PhD (he/him/his)
Senior Research Pharmacologist
US Food and Drug Administration
Silver Spring, Maryland, United States- MA
Md Ashraf Uz Zaman, Ph.D.
ORISE Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States - AA
Ann-Marie Ako-Adounvo, Ph.D.
STAFF FELLOW
US Food and Drug Administration
Silver Spring, Maryland, United States - MK
Megan Kelchen, Ph.D.
SENIOR PHARMACOLOGIST
US Food and Drug Administration
Silver Spring, Maryland, United States 
Priyanka Ghosh, PhD
Lead pharmacologist
US Food and Drug Administration
Silver Spring, Maryland, United States- MA
Muhammad Ashraf, Ph.D.
SUPERVISORY CHEMIST
US Food and Drug Administration
Silver Spring, Maryland, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Microscopic images of azelaic acid gel (lab manufactured formulations and Finacea (azelaic acid) topical gel, 15%) were captured at multiple magnifications after staining with various oil soluble dyes to visualize the oil phase and determine its droplet size distribution. Morphologically directed Raman spectroscopy (MDRS) were also utilized to identify various phases of the gel and to evaluate particle size distribution (PSD) of dispersed solid phase, respectively. The formulations were mechanically separated into various phases to assess phase volumes and drug distribution. A multi-arm in vitro permeation test (IVPT) study (2 donors, 4 replicates per donor) was designed to evaluate the contribution of the oil phase to the permeation of azelaic across human cadaver skin samples. In this IVPT study, azelaic acid permeation from lab manufactured gel formulation containing both oil and aqueous phase was compared with that from gel formulation containing only aqueous phase. The IVPT study also included arms using lab manufactured formulations to elucidate the role of solid particles on overall flux of azelaic acid permeation. The release of azelaic acid from these gel formulations at the early timepoints was also investigated.
Results: Microscopic images and MDRS analysis unveiled the critical microstructural attributes, namely phase status and PSD parameters, of the dispersed phases in the drug product (Figure 1A). Among various dyes investigated, 8-Anilino-1-naphthalenesulfonic acid ammonium salt (ANSA) stained the oil droplets for visual observation; however, it was challenging to determine the droplet size distribution due to their aggregation during the handling procedure for visualization. The results of the mass balance analysis revealed that 76% of azelaic acid is present as a dispersed solid state with a wide PSD, D10, D50, and D90 of 0.58, 1.03, and 3.98 µm, respectively. Minimal distribution of azelaic acid to the dispersed oily phase was observed, which in turn constituted less than 2% w/w of the gel formulation, based on the observations in the current study. Preliminary results from the multi-arm IVPT study suggests that the dispersed solid phase of azelaic acid appears to influence the initial permeation flux (Figure 1B). The preliminary release results suggest that PSD of solid phase influenced the initial drug release rate.
Conclusion: This evaluation scheme and results enhanced our understanding of the performance of azelaic acid gel upon topical application. The identified critical microstructure attributes, namely phase status, PSD, drug release and permeation, may be useful along with other formulation attributes in comparing the performance of gel formulations of azelaic acid for topical applications.
Acknowledgements: This project is supported by an appointment (Md AshrafUzZaman) to the Research Participation Program at the FDA Office of Research and Standards, Office of Generic Drugs, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and FDA. The views expressed in this poster abstract do not reflect the official policies of the U.S. FDA or the U.S. Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the U.S. Government.
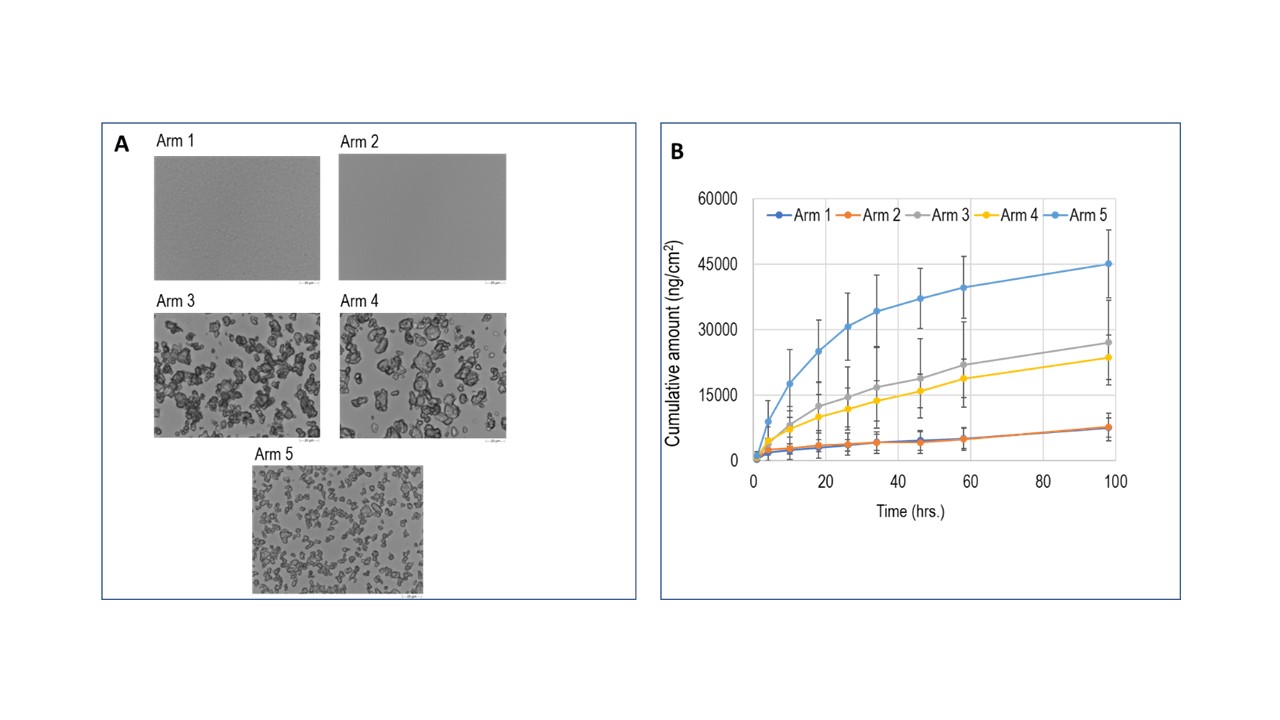 Figure 1: Microscopic images (A) and permeation profiles (B) of azelaic acid gel formulations across human cadaver skin samples (n=4); Arm 1: Aqueous and oil phase saturated with dissolved azelaic acid; Arm 2: Aqueous phase saturated with dissolved azelaic acid; Arm 3: Aqueous phase, oil phase, and 15% (w/w) solid azelaic acid dispersed in formulation; Arm 4: Aqueous phase and 15% (w/w) solid azelaic acid dispersed in formulation; Arm 5: Aqueous phase, oil phase, and 15% (w/w) micronized solid azelaic acid dispersed in formulation.
Figure 1: Microscopic images (A) and permeation profiles (B) of azelaic acid gel formulations across human cadaver skin samples (n=4); Arm 1: Aqueous and oil phase saturated with dissolved azelaic acid; Arm 2: Aqueous phase saturated with dissolved azelaic acid; Arm 3: Aqueous phase, oil phase, and 15% (w/w) solid azelaic acid dispersed in formulation; Arm 4: Aqueous phase and 15% (w/w) solid azelaic acid dispersed in formulation; Arm 5: Aqueous phase, oil phase, and 15% (w/w) micronized solid azelaic acid dispersed in formulation. 