Formulation and Delivery - Biomolecular
(M1530-09-48) Dual Adjuvanted Ionizable Lipid Nanoparticles for Vaccine Delivery
Monday, October 21, 2024
3:30 PM - 4:30 PM MT
.jpg)
Bishal Misra, MS
Ph. D. candidate
West Virginia University
Morgantown, West Virginia, United States- SB
Sharan Bobbala, Ph.D. (he/him/his)
Assistant Professor
West Virginia University
Morgantown, West Virginia, United States
Presenting Author(s)
Main Author(s)
Purpose: Adjuvants act as agonists of pattern recognition receptors (PRRs) and boost the immune response against pathogens by stimulating antigen-presenting cells (APCs) [1]. PRRs such as toll-like receptor TLR9 in the endosome, and TLR4 present on both the cell membrane and endosome, are two of the sought-after targets in the development of prophylactic and therapeutic vaccines. CpG ODN and Monophosphoryl Lipid A (MPLA) are currently used as adjuvants to activate TLR9 and TLR4 receptors, respectively. Synchronous stimulation of these receptors can generate strong antigen-specific cellular and humoral responses. However, the diverse physicochemical properties of CpG ODN and MPLA adjuvants limit their usage for concurrent administration and delivery. With the recent success of mRNA-based COVID vaccines, ionizable lipid nanoparticles (LNPs) have emerged as promising tools for intracellular delivery of nucleic acid vaccines. Ionizable LNPs possess a neutral charge at physiological pH but become positively charged in low-pH environments, such as in the endolysosomes, to achieve intracellular release of the payloads. In this study, we utilized ionizable LNPs to concurrently deliver negatively charged hydrophilic CpG ODN and hydrophobic MPLA adjuvants precisely to APCs to synchronously stimulate TLR9 and TLR4 receptors.
Methods: Ionizable lipid SM102, along with other helper and structural lipid components, such as 1,2-Distearoyl-sn-glycero-3-PC (DSPC), PEG lipid (DMG-PEG2000), and cholesterol, were dissolved in ethanol to form a lipid mixture. MPLA dissolved in DMSO was added to the ethanolic phase containing the lipid mixture, whereas CpG ODN 2395 was dissolved in an aqueous phase containing sodium citrate buffer (pH 4). The organic and aqueous phases were then rapidly mixed in a confined impingement (CIJ) mixer to form LNPs [2]. The unencapsulated adjuvants and ethanol were removed from the formulation using a 10 kDa dialysis membrane at room temperature. The particle size and surface charge of adjuvant LNPs were determined using dynamic light scattering (DLS) and electrophoretic light scattering (ELS), respectively. The morphology of the LNPs was confirmed using Transmission electron microscopy (TEM). The encapsulation efficiency of CpG ODN was measured by the QuantiFluor® ssDNA System. Raw-BlueTM reporter cells, which are derived from murine RAW 264.7, have chromosomal integration of a SEAP reporter construct that can be induced by NF-ĸB and AP-1 and were utilized to assess the activation of immune cells caused by the adjuvanted LNPs. Bone marrow-derived dendritic cells (BMDCs) were used to analyze cellular surface marker expression following activation with adjuvanted LNPs. Enzyme-Linked Immunosorbent Assay (ELISA) was performed to measure the secretion of the cytokine, Tumor necrosis factor – α (TNF-α) following immune activation.
Results: Adjuvanted ionizable LNPs were monodisperse (PDI < 0.3) with sizes ranging from 120–160 nm and exhibited a neutral surface charge at physiological pH (7.4). The TEM images confirmed the spherical morphology of dual adjuvanted LNPs. Immune cell activation studies with Raw-Blue™ cells indicated that the dual-adjuvanted LNPs showed higher SEAP activity compared to the single adjuvanted LNPs encapsulated with either CpG ODN or MPLA. Dual adjuvanted LNPs significantly enhanced surface marker expression of CD40, CD80, and CD86 on BMDCs as compared to single adjuvanted LNPs. Similarly, the ELISA results showed greater secretion of TNF-α following incubation of BMDCs with dual adjuvanted LNPs.
Conclusion: Ionizable LNPs allowed efficient encapsulation of single or dual adjuvants without affecting their morphological characteristics. Dual adjuvanted LNPs were more effective in stimulating immune cells as compared to single adjuvanted LNPs, demonstrating the synergistic effect of TLR4 and TLR9 stimulation. Taken together, these results suggest that the ionizable LNPs can efficiently deliver adjuvants to immune cells to stimulate stronger antigen-specific immune responses.
References: 1. Pulendran, B., P. S. Arunachalam, and D.T. O’Hagan, Emerging concepts in the science of vaccine adjuvants. Nature Reviews Drug Discovery, 2021. 20(6): p. 454-475.
2. Misra, B., et al., Flash nanoprecipitation assisted self-assembly of ionizable lipid nanoparticles for nucleic acid delivery. Nanoscale, 2024. 16(14): p. 6939-6948.
Acknowledgements: Supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme LLC. The opinions expressed in this abstract are those of the authors and do not necessarily represent those of Merck Sharp & Dohme LLC
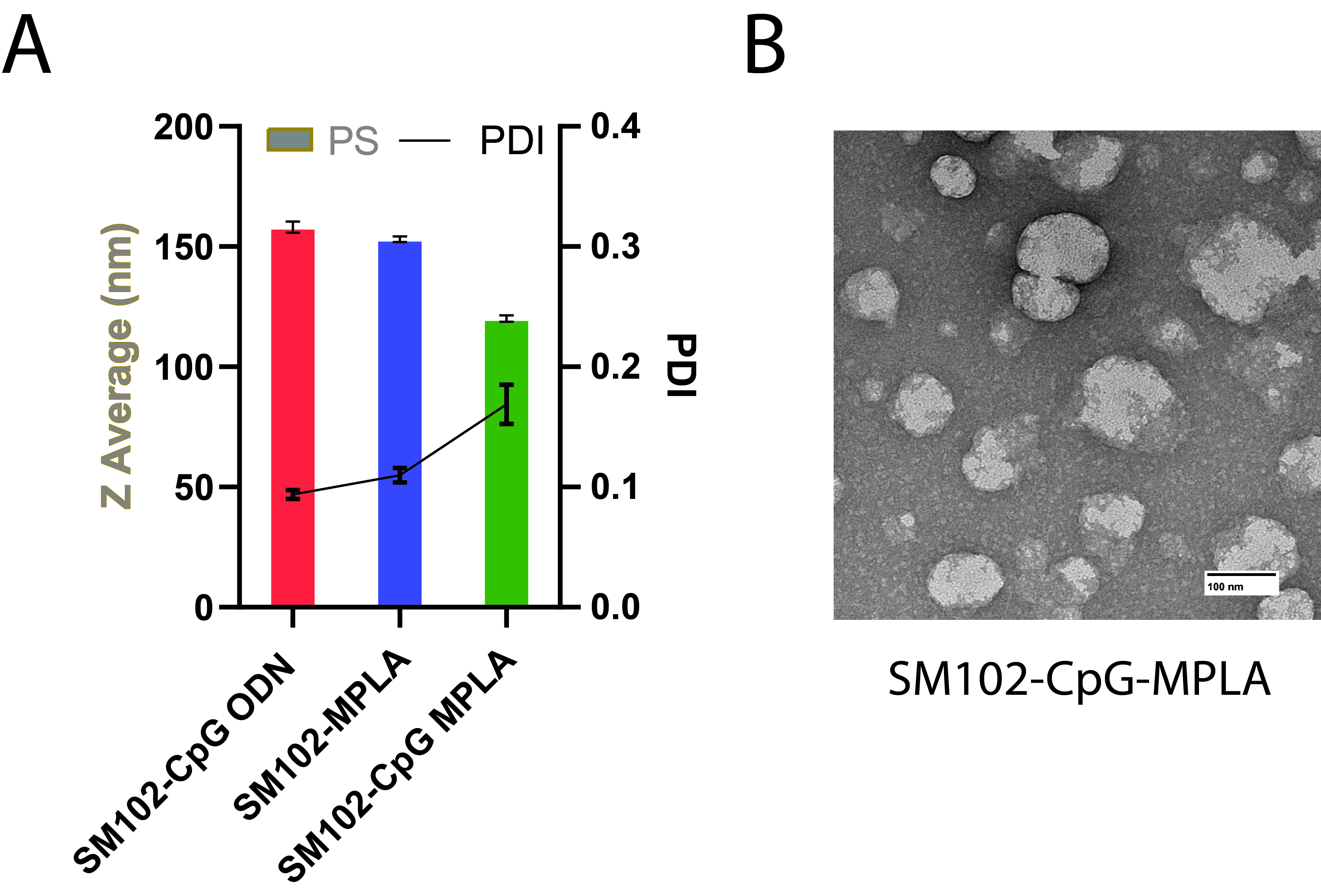 Fig. 1. Morphological characterization of adjuvanted LNPs containing ionizable lipid SM102. A) The particle size of the LNPs measured using dynamic light scattering and the hydrodynamic diameter is reported as Z average (nm). Data are presented as the mean ± SD (n=4). B) Negative stained TEM images of dual adjuvanted SM102-CpG-MPLA LNPs. The scale bar is 100 nm.
Fig. 1. Morphological characterization of adjuvanted LNPs containing ionizable lipid SM102. A) The particle size of the LNPs measured using dynamic light scattering and the hydrodynamic diameter is reported as Z average (nm). Data are presented as the mean ± SD (n=4). B) Negative stained TEM images of dual adjuvanted SM102-CpG-MPLA LNPs. The scale bar is 100 nm.
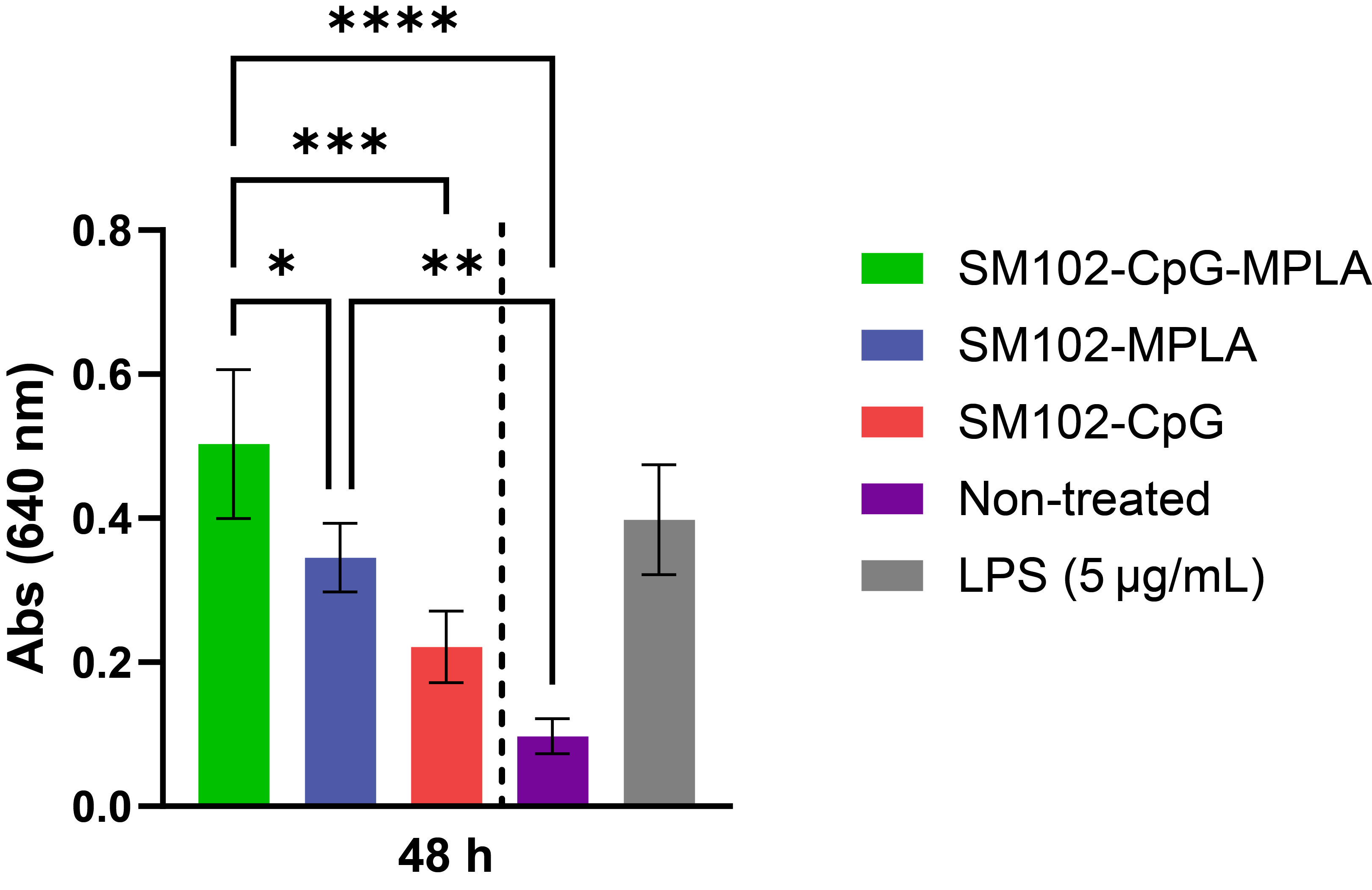 Fig. 2. Secreted embryonic alkaline phosphatase (SEAP) activity of CpG ODN (2.5 µg/mL), MPLA (1.25 µg/mL), and CpG-MPLA (2.5 µg/mL of CpG ODN and 1.25 µg/mL of MPLA) loaded SM102 LNPs after 48 hours of treatment with Raw-Blue™ Cells. Y-axis indicates absorption intensity at 640 nm, directly proportional to the SEAP activity of the samples containing Adjuvants in LNPs. Data are presented as the mean ± SD (n=4). Significant differences between each treatment group were determined by one-way ANOVA with Tukey’s multiple comparison test, *p = 0.0373, **p = 0.0024, ***p = 0.0003, ****p < 0.0001.
Fig. 2. Secreted embryonic alkaline phosphatase (SEAP) activity of CpG ODN (2.5 µg/mL), MPLA (1.25 µg/mL), and CpG-MPLA (2.5 µg/mL of CpG ODN and 1.25 µg/mL of MPLA) loaded SM102 LNPs after 48 hours of treatment with Raw-Blue™ Cells. Y-axis indicates absorption intensity at 640 nm, directly proportional to the SEAP activity of the samples containing Adjuvants in LNPs. Data are presented as the mean ± SD (n=4). Significant differences between each treatment group were determined by one-way ANOVA with Tukey’s multiple comparison test, *p = 0.0373, **p = 0.0024, ***p = 0.0003, ****p < 0.0001.
.jpg) Fig. 3. Analysis of surface marker expression and secreted TNF-α levels. BMDCs were treated with SM102-CpG (10 µg/mL), SM102-MPLA (5 µg/mL) and SM102-CpG-MPLA (10 µg/mL of CpG and 5 µg/mL MPLA) for 24 h. After that surface marker expressions of A) CD40, B) CD80 and C) CD86 were measured using flow cytometry analysis and D) Supernatants were measured for TNF-α using ELISA. Data are presented as the mean ± SD (n=4). Significant differences between each treatment group were determined by one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Fig. 3. Analysis of surface marker expression and secreted TNF-α levels. BMDCs were treated with SM102-CpG (10 µg/mL), SM102-MPLA (5 µg/mL) and SM102-CpG-MPLA (10 µg/mL of CpG and 5 µg/mL MPLA) for 24 h. After that surface marker expressions of A) CD40, B) CD80 and C) CD86 were measured using flow cytometry analysis and D) Supernatants were measured for TNF-α using ELISA. Data are presented as the mean ± SD (n=4). Significant differences between each treatment group were determined by one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Methods: Ionizable lipid SM102, along with other helper and structural lipid components, such as 1,2-Distearoyl-sn-glycero-3-PC (DSPC), PEG lipid (DMG-PEG2000), and cholesterol, were dissolved in ethanol to form a lipid mixture. MPLA dissolved in DMSO was added to the ethanolic phase containing the lipid mixture, whereas CpG ODN 2395 was dissolved in an aqueous phase containing sodium citrate buffer (pH 4). The organic and aqueous phases were then rapidly mixed in a confined impingement (CIJ) mixer to form LNPs [2]. The unencapsulated adjuvants and ethanol were removed from the formulation using a 10 kDa dialysis membrane at room temperature. The particle size and surface charge of adjuvant LNPs were determined using dynamic light scattering (DLS) and electrophoretic light scattering (ELS), respectively. The morphology of the LNPs was confirmed using Transmission electron microscopy (TEM). The encapsulation efficiency of CpG ODN was measured by the QuantiFluor® ssDNA System. Raw-BlueTM reporter cells, which are derived from murine RAW 264.7, have chromosomal integration of a SEAP reporter construct that can be induced by NF-ĸB and AP-1 and were utilized to assess the activation of immune cells caused by the adjuvanted LNPs. Bone marrow-derived dendritic cells (BMDCs) were used to analyze cellular surface marker expression following activation with adjuvanted LNPs. Enzyme-Linked Immunosorbent Assay (ELISA) was performed to measure the secretion of the cytokine, Tumor necrosis factor – α (TNF-α) following immune activation.
Results: Adjuvanted ionizable LNPs were monodisperse (PDI < 0.3) with sizes ranging from 120–160 nm and exhibited a neutral surface charge at physiological pH (7.4). The TEM images confirmed the spherical morphology of dual adjuvanted LNPs. Immune cell activation studies with Raw-Blue™ cells indicated that the dual-adjuvanted LNPs showed higher SEAP activity compared to the single adjuvanted LNPs encapsulated with either CpG ODN or MPLA. Dual adjuvanted LNPs significantly enhanced surface marker expression of CD40, CD80, and CD86 on BMDCs as compared to single adjuvanted LNPs. Similarly, the ELISA results showed greater secretion of TNF-α following incubation of BMDCs with dual adjuvanted LNPs.
Conclusion: Ionizable LNPs allowed efficient encapsulation of single or dual adjuvants without affecting their morphological characteristics. Dual adjuvanted LNPs were more effective in stimulating immune cells as compared to single adjuvanted LNPs, demonstrating the synergistic effect of TLR4 and TLR9 stimulation. Taken together, these results suggest that the ionizable LNPs can efficiently deliver adjuvants to immune cells to stimulate stronger antigen-specific immune responses.
References: 1. Pulendran, B., P. S. Arunachalam, and D.T. O’Hagan, Emerging concepts in the science of vaccine adjuvants. Nature Reviews Drug Discovery, 2021. 20(6): p. 454-475.
2. Misra, B., et al., Flash nanoprecipitation assisted self-assembly of ionizable lipid nanoparticles for nucleic acid delivery. Nanoscale, 2024. 16(14): p. 6939-6948.
Acknowledgements: Supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme LLC. The opinions expressed in this abstract are those of the authors and do not necessarily represent those of Merck Sharp & Dohme LLC
 Fig. 1. Morphological characterization of adjuvanted LNPs containing ionizable lipid SM102. A) The particle size of the LNPs measured using dynamic light scattering and the hydrodynamic diameter is reported as Z average (nm). Data are presented as the mean ± SD (n=4). B) Negative stained TEM images of dual adjuvanted SM102-CpG-MPLA LNPs. The scale bar is 100 nm.
Fig. 1. Morphological characterization of adjuvanted LNPs containing ionizable lipid SM102. A) The particle size of the LNPs measured using dynamic light scattering and the hydrodynamic diameter is reported as Z average (nm). Data are presented as the mean ± SD (n=4). B) Negative stained TEM images of dual adjuvanted SM102-CpG-MPLA LNPs. The scale bar is 100 nm. Fig. 2. Secreted embryonic alkaline phosphatase (SEAP) activity of CpG ODN (2.5 µg/mL), MPLA (1.25 µg/mL), and CpG-MPLA (2.5 µg/mL of CpG ODN and 1.25 µg/mL of MPLA) loaded SM102 LNPs after 48 hours of treatment with Raw-Blue™ Cells. Y-axis indicates absorption intensity at 640 nm, directly proportional to the SEAP activity of the samples containing Adjuvants in LNPs. Data are presented as the mean ± SD (n=4). Significant differences between each treatment group were determined by one-way ANOVA with Tukey’s multiple comparison test, *p = 0.0373, **p = 0.0024, ***p = 0.0003, ****p < 0.0001.
Fig. 2. Secreted embryonic alkaline phosphatase (SEAP) activity of CpG ODN (2.5 µg/mL), MPLA (1.25 µg/mL), and CpG-MPLA (2.5 µg/mL of CpG ODN and 1.25 µg/mL of MPLA) loaded SM102 LNPs after 48 hours of treatment with Raw-Blue™ Cells. Y-axis indicates absorption intensity at 640 nm, directly proportional to the SEAP activity of the samples containing Adjuvants in LNPs. Data are presented as the mean ± SD (n=4). Significant differences between each treatment group were determined by one-way ANOVA with Tukey’s multiple comparison test, *p = 0.0373, **p = 0.0024, ***p = 0.0003, ****p < 0.0001. .jpg) Fig. 3. Analysis of surface marker expression and secreted TNF-α levels. BMDCs were treated with SM102-CpG (10 µg/mL), SM102-MPLA (5 µg/mL) and SM102-CpG-MPLA (10 µg/mL of CpG and 5 µg/mL MPLA) for 24 h. After that surface marker expressions of A) CD40, B) CD80 and C) CD86 were measured using flow cytometry analysis and D) Supernatants were measured for TNF-α using ELISA. Data are presented as the mean ± SD (n=4). Significant differences between each treatment group were determined by one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Fig. 3. Analysis of surface marker expression and secreted TNF-α levels. BMDCs were treated with SM102-CpG (10 µg/mL), SM102-MPLA (5 µg/mL) and SM102-CpG-MPLA (10 µg/mL of CpG and 5 µg/mL MPLA) for 24 h. After that surface marker expressions of A) CD40, B) CD80 and C) CD86 were measured using flow cytometry analysis and D) Supernatants were measured for TNF-α using ELISA. Data are presented as the mean ± SD (n=4). Significant differences between each treatment group were determined by one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.