Formulation and Delivery - Chemical
(M1530-10-56) Enhancing the Solubilization and Permeation of Fenofibrate: A Comparative Study of Free Form, PEG and SEDDS Formulations
- MJ
Ming Ji, Ph.D.
Scientist III
BASF Corporation
Tarrytown, New York, United States 
Nitin K. Swarnakar, PhD (he/him/his)
NA Applications Lab Manager
BASF Corp
Tarrytown, New York, United States- MJ
Ming Ji, Ph.D.
Scientist III
BASF Corporation
Tarrytown, New York, United States - JW
Jarom D. Webster, MS
Staff Scientist, R&D
Thermo Fisher Scientific
Cincinnati, Ohio, United States - AC
Andrea Cady, Ph.D.
Sr. Manager, Formulation and Process Development
Thermo Fisher Scientific
Cincinnati, Ohio, United States 
Ramesh Muttavarapu, MS
Global SME - Technical & Scieitifc Affairs
Thermo Fisher Scientific
Cincinnati, Ohio, United States- JW
Jason S. Wood, Ph.D.
Technical Services Manager
BASF Corporation
Tarrytown, New York, United States 
Sandip Tiwari, PhD (he/him/his)
Head of Technical Services - Pharma Solutions
BASF Corporation
Tarrytown, New York, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: The SEDDS preconcentrates were prepared using the concentrations of excipients shown in Table 1. Drug-loaded SEDDS and PEG 400 were produced by weighing the API directly into glass vials and adding the SEDDS pre-concentrate or PEG 400 and filled into hard gelatin capsules of size “00”. The final drug load in the formulations corresponded to 50-mg. The dissolution of the three formulations and flux across membrane was tested using MacroFLUXTM (Pion Inc., Billerica, MA). The acceptor compartment is integrated with a permeable membrane, an overhead stirrer, and a fiber optic UV probe (10 mm path length), which is inserted into the 1000 mL vessel of a USP II apparatus (Erweka DT 126 Dissolution Tester, Heusenstamm Germany). The artificial Gastro-intestinal (GIT) mimicking membrane was formed by 50 µL of GIT-0 lipid solution on the filter support [hydrophobic polyvinylidenfluoride (PVDF), 0.45 µm pore size, 3.8 cm2]. For permeation experiment, 10 mL of pH 7.4 acceptor sink buffer (ASB) at stirring speed 500 rpm in acceptor compartment and 1000 mL of pH 6.5 fasted state simulated intestinal fluid version 2 (FaSSIF V 2) at stirring speed of 75 rpm in donor compartment. The integrated fiber-optic UV probes were positioned in the donor (λ 320 nm) and acceptor compartments (λ 320 nm). The flux (J) across the membrane was calculated using the following equation:
J (t) = dm/Adtwhere (m) is the the amount of drug crossing a unit area (A) perpendicular to its flow per unit time (t). The flux values of formulations were calculated from the linear portion of the permeation profile.
Results: Based on initial solubilization studies, the SEDDS formulation showed a higher drug solubilization i.e. 70 mg/g compared to 30 mg/g in the PEG formulation. The demonstrated superior solubility of the SEDDS formulation makes it a promising option for drug delivery of water-insoluble drugs like fenofibrate for better clinical performance. The free fenofibrate formulation demonstrated a low dissolution of 2 % and a permeation rate of 0.009 ug/cm2/h. The PEG formulation exhibited a 100% dissolution within 5 h and a linear steady state flux of 0.39 ug/sec cm2 (between 0.5 h to 5 h However, once the drug reached saturation, fenofibrate showed precipitation kinetic leading to the high variability in the solubilized drug. After 18 hours, a drug dissolution reduced to 10 % (Figure 1A and Figure 2). This indicates the necessity of a drug in solution requiring a suitable crystallization inhibitor to maintain kinetic stability of drug in the PEG based formulation. Conversely, the SEDDS formulation demonstrated a high dissolution of drug 100% within 5 h and a steady state flux of 0.32 ug/sec cm2 (between 0.5 h to 6 h) with no change in dissolution levels after 18 hours (Figure 1B and Figure 2). This suggests excellent solubilization and thermodynamic stability by SEDDS formulation, indicating a better absorption window for fenofibrate permeation. The formation of secondary structures in SEDDS digestion needs further investigation to understand its solubilization potential in vivo. It is interesting to know that the SEDDS and PEG showed almost similar flux levels despite of different solubilization behavior in the dissolution performance. This could be explained by saturation of the membrane surface area (3.82 cm2) via drug molecules by both formulations, Additionally, saturation of fenofibrate concentration was observed in the 10 ml of sink buffer after 6-8 hours in the acceptor chamber, leading to a drop in the max flux beyond 8 h. This observation emphasizes the importance of evaluating saturation of membrane flux and sink solution at suitable concentration ranges in the dissolution vessel to fully understand the delivery potential of the formulations and their ranking order.
Conclusion: The PEG 400 and SEDDS formulations demonstrated significantly higher permeation rates compared to the free fenofibrate formulation; however, dissolution performance of the SEDDS formulation was superior. The study highlights the importance of kinetic and thermodynamic stability in drug formulation containing high concentration of solubilizers/stabilizers. During permeation studies, the critical consideration should be given to evaluate the membrane and sink buffer saturation because the drug concentration may exceed the saturation solubility of the free drug in the media. This research highlights the potential of SEDDS in improving the solubilization of water-insoluble drugs like fenofibrate, thereby improving the patient compliance via reducing pill burden, dissolution, and permeability as compared to free drug.
References: [1] https://pubchem.ncbi.nlm.nih.gov/compound/Fenofibrate#section=Drug-Labels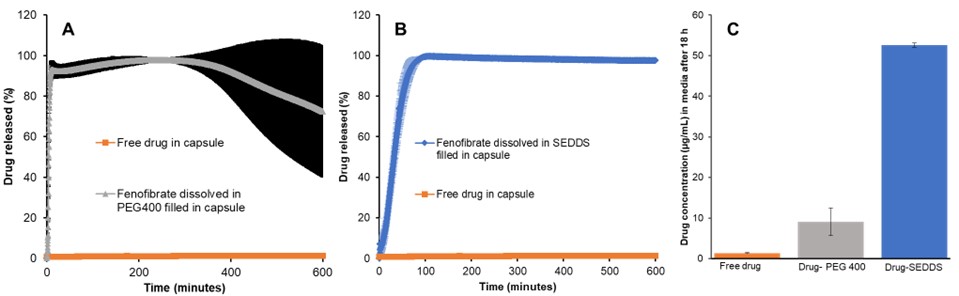 Figure 1: A) Dissolution profile of fenofibrate loaded PEG400 formulation and free drug (up to 10 h). B) Dissolution profile of fenofibrate loaded SEDDS formulation and free drug. C) Bar graph showed drug concentration in media after 18 h of dissolution (value are expressed as mean ± SD)
Figure 1: A) Dissolution profile of fenofibrate loaded PEG400 formulation and free drug (up to 10 h). B) Dissolution profile of fenofibrate loaded SEDDS formulation and free drug. C) Bar graph showed drug concentration in media after 18 h of dissolution (value are expressed as mean ± SD)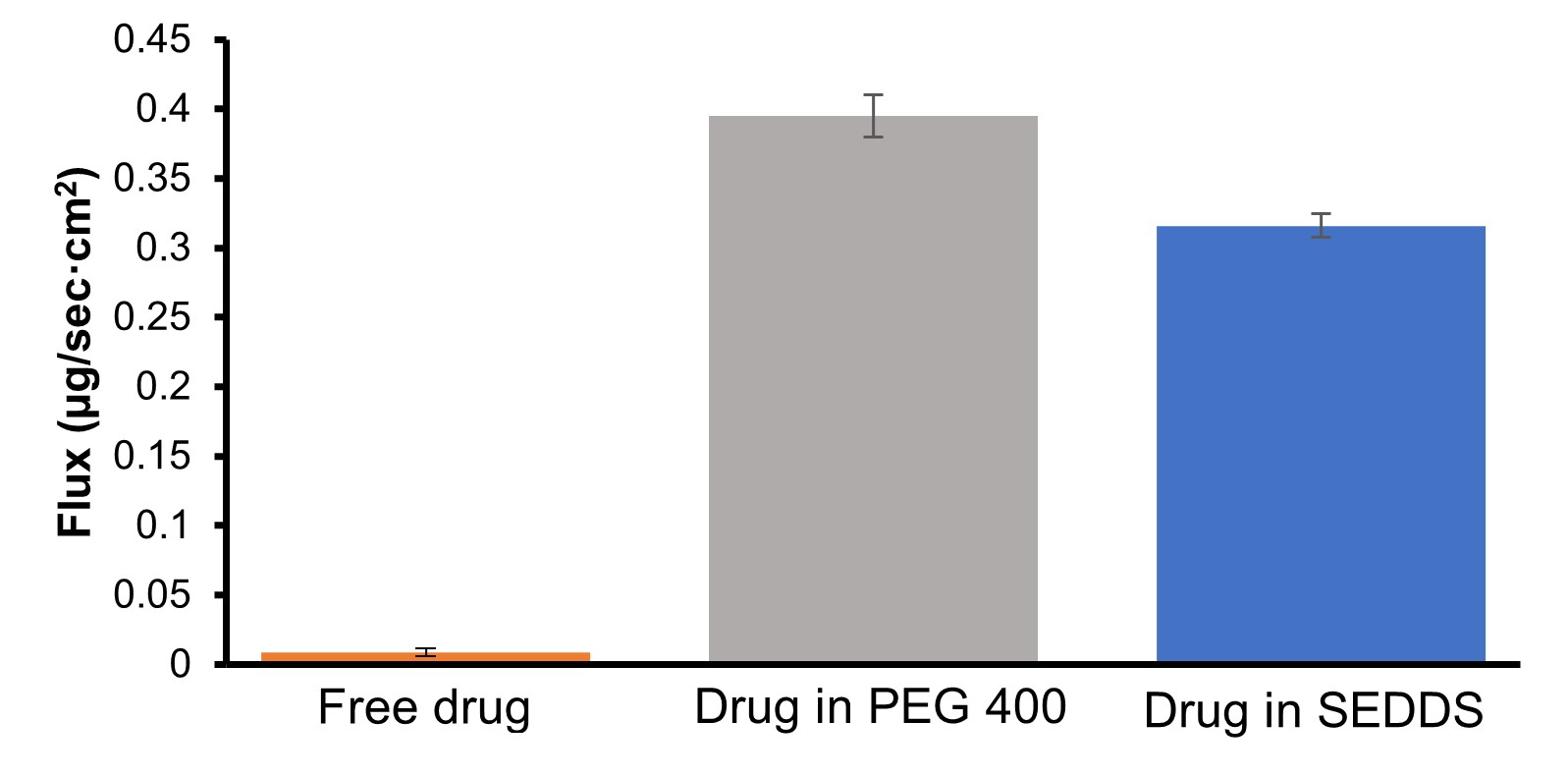 Figure 2: In vitro steady state flux of drug through the GIT-O lipid membrane using different formulations, as measured by Pion’s MacroFLUXTM instrument. (value are expressed as mean ± SD)
Figure 2: In vitro steady state flux of drug through the GIT-O lipid membrane using different formulations, as measured by Pion’s MacroFLUXTM instrument. (value are expressed as mean ± SD)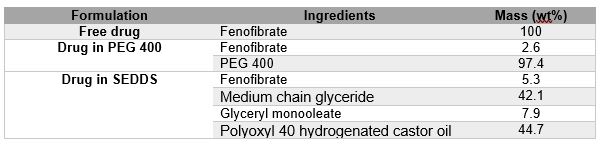 Tablet 1: Type of fenofibrate loaded formulation and compositions.
Tablet 1: Type of fenofibrate loaded formulation and compositions.
