Formulation and Delivery - Chemical
(T1530-01-01) Proven New Sustainable Eco-Friendly Packaging for Challenging Formulation Compositions
Tuesday, October 22, 2024
3:30 PM - 4:30 PM MT

Gemma Casadevall, PhD (she/her/hers)
CEO
Nutraresearch
Hospitalet del llobregat, Catalonia, Spain- JS
Javier Sala, MS
Pilot Plant Manager
Nutraresearch
Hospitalet del llobregat, Catalonia, Spain
Presenting Author(s)
Main Author(s)
Purpose: The global dietary supplements market size was estimated at USD 177.50 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 9.1% from 2024 to 2030. Amongst many challenges facing this revolution in dietary supplements is the commitment to natural-sourced ingredients, sustainable packaging, and clean label formulations, overcomed by the use of pharmaceutical technology techniques, that can prove robust and stable formulations during the shelf-life period. Placing in the market stable and robust formulations is a challenge (and even so when previous stability studies are not compulsory) given the nature of food supplements compositions (naturally sourced, highly variable and sometimes hygroscopic). Thus, challenging stability robustness in terms of not only chemical stability but pharmacothecnical characteristics against temperature and humidity becomes a must. The biggest challenge amongst the new food supplement trends is, on top of their unstable nature to be brought into the market under sustainable packaging, specifically blisters, that can ensure a product that has the final desired quality for the final consumer. As of now, blisters in the market are composed by aluminum or a plastic polymer (PCV, PVDC) that is sealed with aluminum, the material, once sealed cannot be separated again and thus, the residue cannot be recycled properly. This study has focused on comparing stability studies (according to ICH Q1A), with 3 different blister materials (PVC/Alu, PVC/PVDC/Alu, and a sustainable material), that can be recycled since it is plastic/plastic, using for this purpose challenging food supplement formulations chosen from a worse case risk analysis matrix that has taken into account factors such as: hygroscopicity, natural product variability sources, and modified release compositions.
Methods: Formulation composition: Four formulation compositions have been chosen using a worse case risk analysis matrix taking into account factors such as: hygroscopicity, natural product variability sources, and modified release compositions. Manufacturing Process: All materials have been weighed, sieved through a 0.8mm mesh and blended using a bin blender. A direct compression process, in a rotary, 9 station Intimac tablet press has been performed. Afterwards, tablets have been packaged accordingly using either PVC+Alu, PVC/PVDC+Alu and sustainable Blister using an Uhlmann UPS 1020 blister industrial machine. Stability studies: All blisters are placed in climatic chambers according to ICH Q1A guideline recommendations: Storage Conditions 25ºC±2ºC/60% RH±5%; 30ºC±2ºC/65% RH±5%; 40ºC±2ºC/75% RH±5% during 6 month. Formulation Quality Attributes analyzed, and methods used: Tablet Weight (mg) à Internal method, analytical scale. Color à Internal method, Konica Minolta CR-5, equipped with colorimeter. Hardness (Kp)à According to USP < 1217 > Tablet Breaking Force, Pharmatest PTB.311E. Disintegration time (min) à According to USP < 701 > Disintegration, Pharmatest PTZA.1EZ. LOD (%) à According to USP < 731 > Loss on drying, ADAM PMB53. Dissolution Test (Accumulated and Release Rate (%)): According to USP 〈711〉 DISSOLUTION USP Apparatus II. Melatonin and Ashwagandha have been analyzed using HPLC Agilent 1260 according to USP (USP-NF Melatonin Tablets and USP-NF Ashwagandha Root Dry Extract). Data Analysis: JMP statistical software v.17 is used to perform statistical analysis.
Results: Tablet Weight (mg): The weight of the 4 formulations was kept consistent for all timepoints, for all materials and across climatic conditions, with no significant differences (p 0.05) . Color: Color of Melatonin+Extract, Melatonin SR and Ashwagandha tablets was steady during all time points and stability conditions, without differences between the samples studied except for Collagen IR tablets, where color was slightly beige at 40ºC at the 6-month timepoint. Hardness (Kp) and Disintegration Time (min): Hardness and disintegration of the 4 formulations is stable during all the timepoints, for all conditions and packaging studied, with significant differences (p 0.05) between the blisters. LOD (%): LOD of the 4 formulations was stable. There were no significant differences (p 0.05) between the 3 blister packs of different materials of different materials at all condition points. Dissolution Test: The profile of the SR formulations was kept consistent for all timepoints, for all materials and across climatic conditions, with no significant differences (p 0.05).
Conclusion: Food supplements are increasingly entering into the market and with them, the trend of clean label formulations with sustainable packaging is evolving. This becomes a great challenge when formulating. In the present study, four different formulation compositions have been studied under ICH Q1A stability guideline conditions, to evaluate three different materials of blister packs, being one of them an environmental sustainable option. Results obtained, show that the final products (including modified release tablets) keep stable and similar results under the three blister packs. This finding constitutes a great advance when it comes to using sustainable material in challenging formulations (from variable and hygroscopic sources) and solves new global sustainability trends.
.jpg) Risk analysis matrix
Risk analysis matrix
.jpg) Color results collagen tablets
Color results collagen tablets
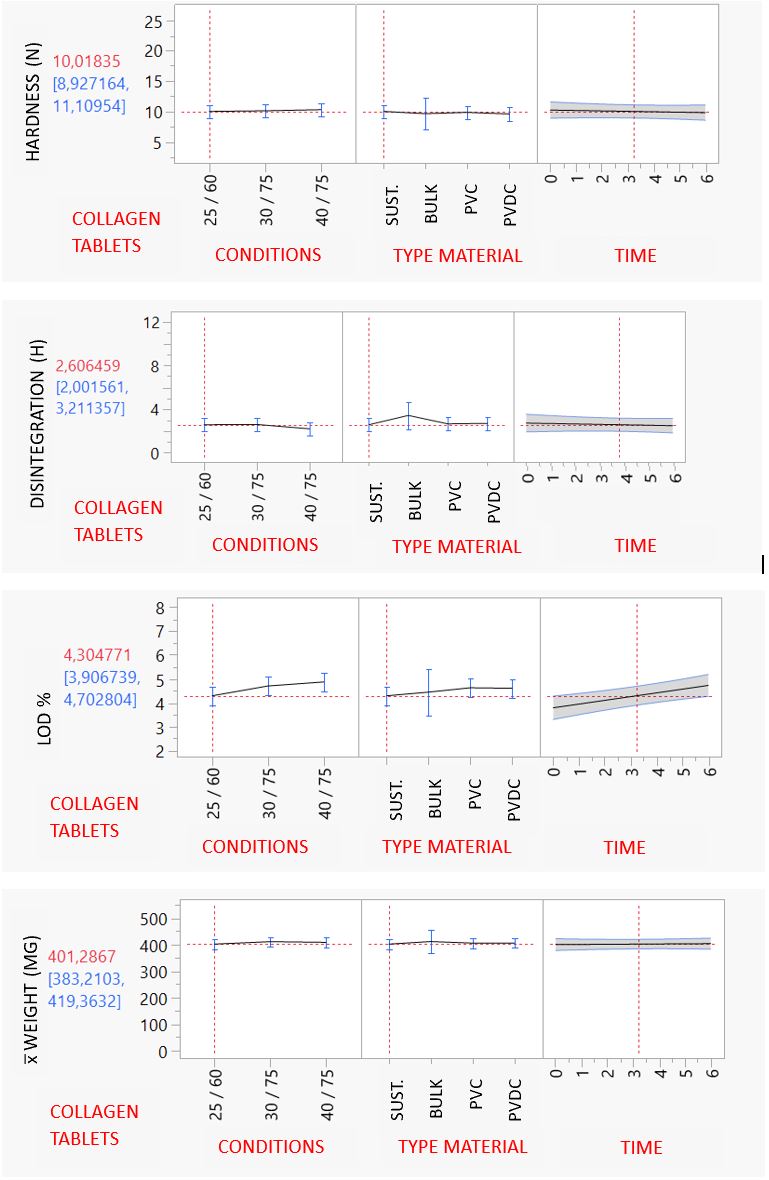 Quality atrtributes results collagen tablets
Quality atrtributes results collagen tablets
Methods: Formulation composition: Four formulation compositions have been chosen using a worse case risk analysis matrix taking into account factors such as: hygroscopicity, natural product variability sources, and modified release compositions. Manufacturing Process: All materials have been weighed, sieved through a 0.8mm mesh and blended using a bin blender. A direct compression process, in a rotary, 9 station Intimac tablet press has been performed. Afterwards, tablets have been packaged accordingly using either PVC+Alu, PVC/PVDC+Alu and sustainable Blister using an Uhlmann UPS 1020 blister industrial machine. Stability studies: All blisters are placed in climatic chambers according to ICH Q1A guideline recommendations: Storage Conditions 25ºC±2ºC/60% RH±5%; 30ºC±2ºC/65% RH±5%; 40ºC±2ºC/75% RH±5% during 6 month. Formulation Quality Attributes analyzed, and methods used: Tablet Weight (mg) à Internal method, analytical scale. Color à Internal method, Konica Minolta CR-5, equipped with colorimeter. Hardness (Kp)à According to USP < 1217 > Tablet Breaking Force, Pharmatest PTB.311E. Disintegration time (min) à According to USP < 701 > Disintegration, Pharmatest PTZA.1EZ. LOD (%) à According to USP < 731 > Loss on drying, ADAM PMB53. Dissolution Test (Accumulated and Release Rate (%)): According to USP 〈711〉 DISSOLUTION USP Apparatus II. Melatonin and Ashwagandha have been analyzed using HPLC Agilent 1260 according to USP (USP-NF Melatonin Tablets and USP-NF Ashwagandha Root Dry Extract). Data Analysis: JMP statistical software v.17 is used to perform statistical analysis.
Results: Tablet Weight (mg): The weight of the 4 formulations was kept consistent for all timepoints, for all materials and across climatic conditions, with no significant differences (p 0.05) . Color: Color of Melatonin+Extract, Melatonin SR and Ashwagandha tablets was steady during all time points and stability conditions, without differences between the samples studied except for Collagen IR tablets, where color was slightly beige at 40ºC at the 6-month timepoint. Hardness (Kp) and Disintegration Time (min): Hardness and disintegration of the 4 formulations is stable during all the timepoints, for all conditions and packaging studied, with significant differences (p 0.05) between the blisters. LOD (%): LOD of the 4 formulations was stable. There were no significant differences (p 0.05) between the 3 blister packs of different materials of different materials at all condition points. Dissolution Test: The profile of the SR formulations was kept consistent for all timepoints, for all materials and across climatic conditions, with no significant differences (p 0.05).
Conclusion: Food supplements are increasingly entering into the market and with them, the trend of clean label formulations with sustainable packaging is evolving. This becomes a great challenge when formulating. In the present study, four different formulation compositions have been studied under ICH Q1A stability guideline conditions, to evaluate three different materials of blister packs, being one of them an environmental sustainable option. Results obtained, show that the final products (including modified release tablets) keep stable and similar results under the three blister packs. This finding constitutes a great advance when it comes to using sustainable material in challenging formulations (from variable and hygroscopic sources) and solves new global sustainability trends.
.jpg) Risk analysis matrix
Risk analysis matrix.jpg) Color results collagen tablets
Color results collagen tablets Quality atrtributes results collagen tablets
Quality atrtributes results collagen tablets