Formulation and Delivery - Chemical
(W1130-09-49) Tyrosine Kinase Inhibitor Loaded Inhalable Liposomal Formulation for Non-Small Cell Lung Cancer
Wednesday, October 23, 2024
11:30 AM - 12:30 PM MT

Apoorva Daram, M.S. (she/her/hers)
Doctoral Student
St. John's University
Queens, New York, United States
Apoorva Daram, M.S. (she/her/hers)
Doctoral Student
St. John's University
Queens, New York, United States- RC
Ruginn Catarata, MS
Research Technician
St. John's University
Queens, New York, United States 
Nitesh K. Kunda, Ph.D.
Assistant Professor
St. John's University
Jamacia, New York, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Lung cancer is the leading cause of cancer mortalities globally, of which non-small cell lung cancer accounts for most of the cases. Tyrosine Kinase Inhibitors (TKI) have been extensively used to treat non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) overexpression. There are multiple TKIs that have been Food and Drug Administration (FDA) approved and are currently on the market for NSCLC patients bearing EGFR mutations. However, within a few months of treatment most patients develop resistance due to mutations in EGFR. Furthermore, resistance to EGFR-TKI therapy due to triple mutations (sensitizing mutations, T790M and C797S) will become a significant challenge. Therefore, there is a need to overcome the resistance, which can be effectively achieved by targeting the EGFR with therapies that will overcome C797S mutations. Recently, an investigational drug, NK1221, has shown efficacy against NSCLC cells bearing C797S mutation. In this study, NK1221 was encapsulated into liposomes to enhance drug delivery. Further, pulmonary administration of liposomes was utilized for safe and efficacious delivery of the drug to the target site i.e. the lungs, limiting systemic toxicity.
Methods: In the present study, NK1221 liposomes were prepared by the thin-film hydration method (via passive and active loading) and ethanolic injection method. For both the approaches, varying molar ratios of lipids DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) and cholesterol were utilized. The prepared liposomes were characterized for particle size, polydispersity, zeta potential, entrapment efficiency and drug loading. To further characterize the solid state of the entrapped drug, differential scanning calorimetry (DSC) studies were performed. The aerosolization performance of the formulation was evaluated using a Pari LC® nebulizer device on a Next Generation Impactor (NGITM) operated at a flow rate of 15 L/min for 4 minutes. The in vitro cytotoxicity was evaluated using WST assay on the BaF3 cell line transfected with EGFRL858R/T970M/C797S mutations. Briefly, the BaF3 cells were treated with various concentrations of free drug and drug loaded liposomes for 48 h before the addition of WST reagents. In addition, all the treatments were supplemented with anti-EGFR antibody to potentiate the effect of NK1221. The stability studies for the liposomes are being performed upon storage at refrigerated conditions (4˚C).
Results: The liposomes were prepared by various techniques to achieve high entrapment efficiency and drug loading. The liposomes prepared via thin-film hydration and ethanolic injection methods exhibited different properties. The liposomes prepared by the thin-film hydration method demonstrated a lower entrapment efficiency (~7.5% w/w for active loading and ~4.5% w/w for passive loading) and drug loading (~6.5 µg/mg of lipid for active loading and ~3.5 µg/mg of lipid for passive loading) compared to the formulation prepared using the ethanolic injection method (~67% w/w for entrapment efficiency and ~57 µg/mg of lipid for drug loading). These liposomes (NLPE) prepared using the ethanolic injection method exhibited a particle size of 182.37 ± 6.09 nm with a polydispersity index of 0.25 ± 0.01 and a zeta potential of -7.61 ± 1.08 mV (Fig. 1). The disappearance of the drug melting peak (~ 235 ℃) in the DSC thermograms for drug-loaded liposomes (NLPE) when compared to the free drug indicated successful encapsulation of drug into the liposomes (Fig. 2A). Additionally, in vitro aerosolization studies performed using NGI demonstrated a mass median aerodynamic diameter of 4.24 ± 0.09 µm and a high fine particle fraction of 79.87 ± 2.89% w/w, implying the deposition of the formulation in the respiratory airways (Fig. 2B). The in vitro studies on BaF3 cells displayed higher cytotoxicity for NK1221 liposomes (NLPE) with ~1.8 reduction in inhibitory concentration (IC50) value compared to free drug (Fig. 3). The stability studies are currently ongoing, but the drug-loaded liposomes have maintained their particle size upon storage at 4 ℃ for up to 2 weeks.
Conclusion: Our results show that inhalable NK1221 liposomes can overcome EGFR T790M- and C797S-induced resistance and potentially contribute to greater anti-tumor efficacy for non-small cell lung cancer treatment.
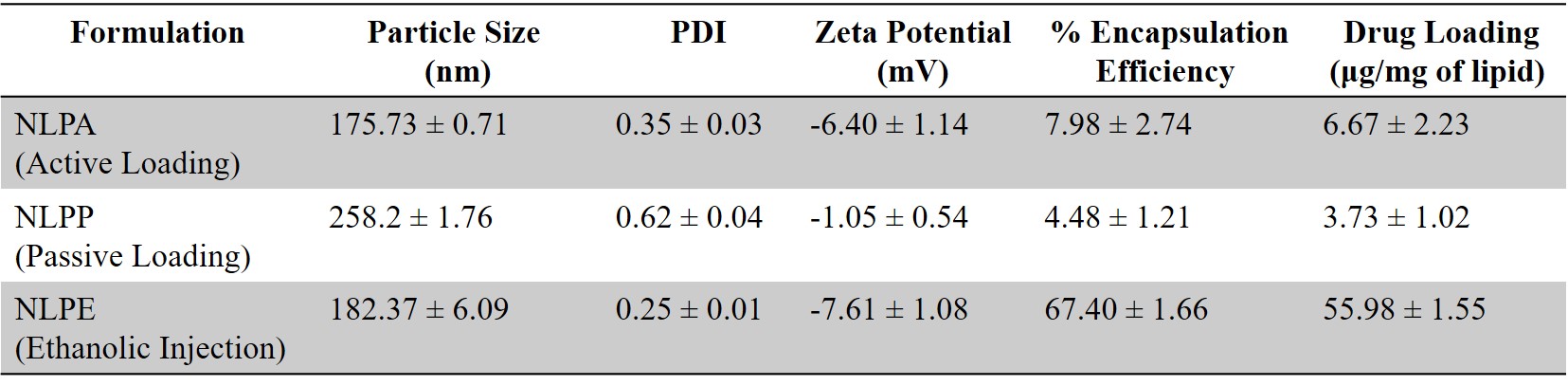 Figure 1. Formulation development. Physiochemical characterization of NK1221 Liposomes. Data represents mean ± SD (n > 3).
Figure 1. Formulation development. Physiochemical characterization of NK1221 Liposomes. Data represents mean ± SD (n > 3).
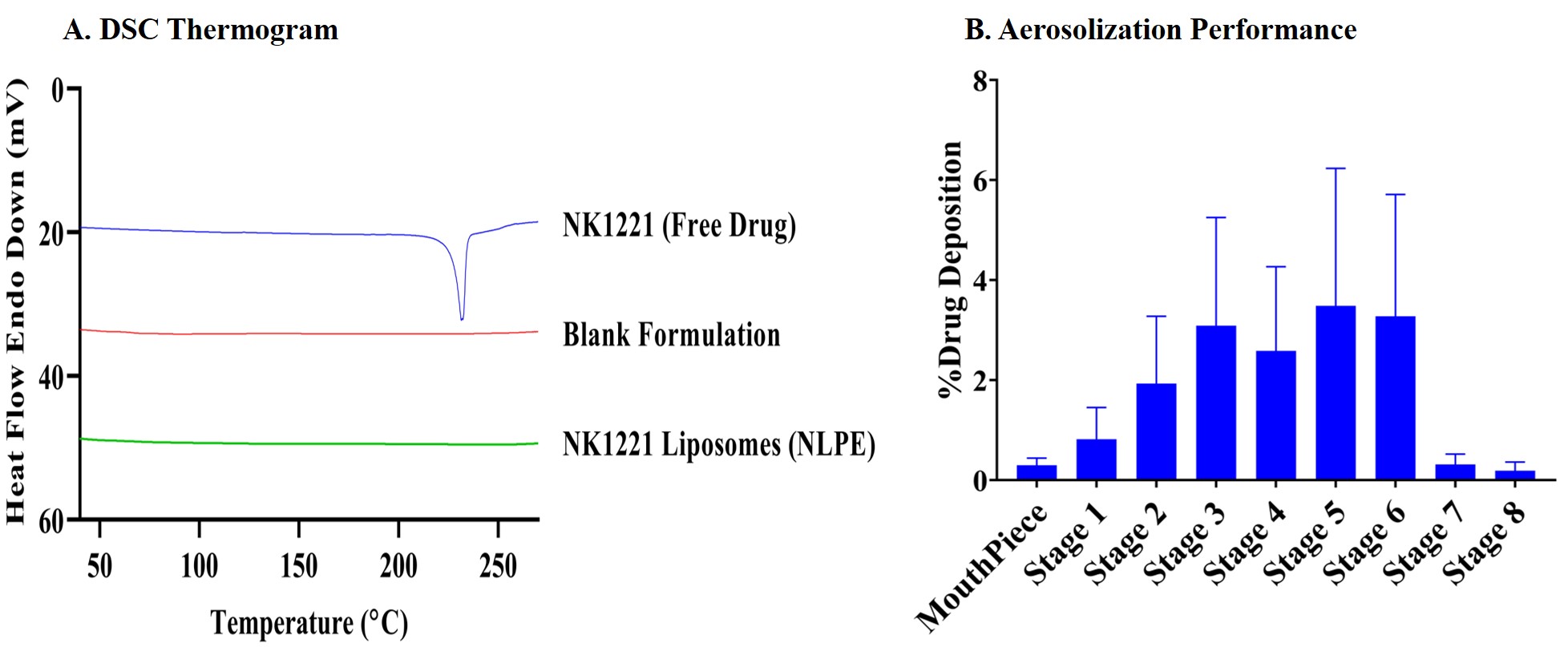 Figure 2. Liposomal characterization. A. The DSC thermogram of NK1221, blank formulation, and NK1221 liposomes (NLPE). B. In vitro aerosol deposition profile represented as the percentage of drug deposited on each stage of Next Generation Impactor (NGI). Data represents mean ± SD (n = 3)
Figure 2. Liposomal characterization. A. The DSC thermogram of NK1221, blank formulation, and NK1221 liposomes (NLPE). B. In vitro aerosol deposition profile represented as the percentage of drug deposited on each stage of Next Generation Impactor (NGI). Data represents mean ± SD (n = 3)
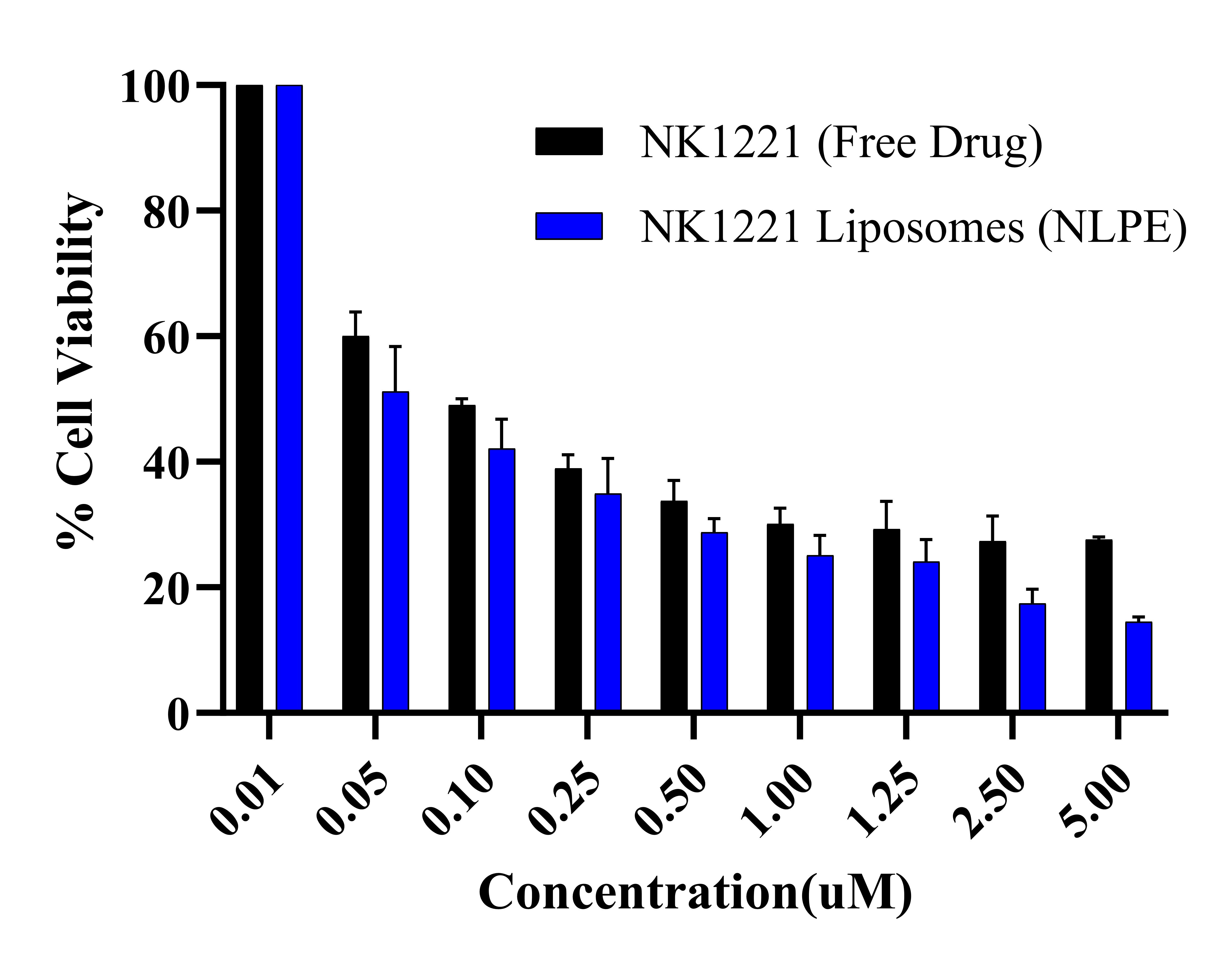 Figure 3. Cytotoxicity Assay. Cytotoxicity studies after 72 h treatment with NK1221, and NK1221 liposomes determined using WST assay in BaF3 cell line.
Figure 3. Cytotoxicity Assay. Cytotoxicity studies after 72 h treatment with NK1221, and NK1221 liposomes determined using WST assay in BaF3 cell line.
Methods: In the present study, NK1221 liposomes were prepared by the thin-film hydration method (via passive and active loading) and ethanolic injection method. For both the approaches, varying molar ratios of lipids DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) and cholesterol were utilized. The prepared liposomes were characterized for particle size, polydispersity, zeta potential, entrapment efficiency and drug loading. To further characterize the solid state of the entrapped drug, differential scanning calorimetry (DSC) studies were performed. The aerosolization performance of the formulation was evaluated using a Pari LC® nebulizer device on a Next Generation Impactor (NGITM) operated at a flow rate of 15 L/min for 4 minutes. The in vitro cytotoxicity was evaluated using WST assay on the BaF3 cell line transfected with EGFRL858R/T970M/C797S mutations. Briefly, the BaF3 cells were treated with various concentrations of free drug and drug loaded liposomes for 48 h before the addition of WST reagents. In addition, all the treatments were supplemented with anti-EGFR antibody to potentiate the effect of NK1221. The stability studies for the liposomes are being performed upon storage at refrigerated conditions (4˚C).
Results: The liposomes were prepared by various techniques to achieve high entrapment efficiency and drug loading. The liposomes prepared via thin-film hydration and ethanolic injection methods exhibited different properties. The liposomes prepared by the thin-film hydration method demonstrated a lower entrapment efficiency (~7.5% w/w for active loading and ~4.5% w/w for passive loading) and drug loading (~6.5 µg/mg of lipid for active loading and ~3.5 µg/mg of lipid for passive loading) compared to the formulation prepared using the ethanolic injection method (~67% w/w for entrapment efficiency and ~57 µg/mg of lipid for drug loading). These liposomes (NLPE) prepared using the ethanolic injection method exhibited a particle size of 182.37 ± 6.09 nm with a polydispersity index of 0.25 ± 0.01 and a zeta potential of -7.61 ± 1.08 mV (Fig. 1). The disappearance of the drug melting peak (~ 235 ℃) in the DSC thermograms for drug-loaded liposomes (NLPE) when compared to the free drug indicated successful encapsulation of drug into the liposomes (Fig. 2A). Additionally, in vitro aerosolization studies performed using NGI demonstrated a mass median aerodynamic diameter of 4.24 ± 0.09 µm and a high fine particle fraction of 79.87 ± 2.89% w/w, implying the deposition of the formulation in the respiratory airways (Fig. 2B). The in vitro studies on BaF3 cells displayed higher cytotoxicity for NK1221 liposomes (NLPE) with ~1.8 reduction in inhibitory concentration (IC50) value compared to free drug (Fig. 3). The stability studies are currently ongoing, but the drug-loaded liposomes have maintained their particle size upon storage at 4 ℃ for up to 2 weeks.
Conclusion: Our results show that inhalable NK1221 liposomes can overcome EGFR T790M- and C797S-induced resistance and potentially contribute to greater anti-tumor efficacy for non-small cell lung cancer treatment.
 Figure 1. Formulation development. Physiochemical characterization of NK1221 Liposomes. Data represents mean ± SD (n > 3).
Figure 1. Formulation development. Physiochemical characterization of NK1221 Liposomes. Data represents mean ± SD (n > 3).  Figure 2. Liposomal characterization. A. The DSC thermogram of NK1221, blank formulation, and NK1221 liposomes (NLPE). B. In vitro aerosol deposition profile represented as the percentage of drug deposited on each stage of Next Generation Impactor (NGI). Data represents mean ± SD (n = 3)
Figure 2. Liposomal characterization. A. The DSC thermogram of NK1221, blank formulation, and NK1221 liposomes (NLPE). B. In vitro aerosol deposition profile represented as the percentage of drug deposited on each stage of Next Generation Impactor (NGI). Data represents mean ± SD (n = 3) Figure 3. Cytotoxicity Assay. Cytotoxicity studies after 72 h treatment with NK1221, and NK1221 liposomes determined using WST assay in BaF3 cell line.
Figure 3. Cytotoxicity Assay. Cytotoxicity studies after 72 h treatment with NK1221, and NK1221 liposomes determined using WST assay in BaF3 cell line.