Formulation and Delivery - Chemical
(T0930-09-51) Enhanced PBPK-Based In Vitro to In Vivo Extrapolation Method to Support the Development of Pulmonary Drug Products
- JM
James Mullin, MS
Sr. Principal Scientist
Simulations Plus, Inc.
Lancaster, California, United States - JM
James Mullin, MS
Sr. Principal Scientist
Simulations Plus, Inc.
Lancaster, California, United States - ML
Maxime Le Merdy, Ph.D.
Director, PBPK Research & Collaborations
Simulations Plus, Inc.
Lancaster, California, United States - SC
Steven Chopski, Ph.D.
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States 
Ross Walenga, Ph.D.
Sr. Chemical Engineer
US Food and Drug Administration
Silver Spring, Maryland, United States- EB
Elizabeth Bielski, Ph.D. (she/her/hers)
Senior Pharmacologist
US Food and Drug Administration
Silver Springs, Maryland, United States - SB
Susan Boc, Ph.D. (she/her/hers)
Pharmacuetical Scientist
US Food and Drug Administration
Silver Spring, Maryland, United States - AC
Andrew Clerman, M.D.
Senior Physician
US Food and Drug Administration
Silver Spring, Maryland, United States - BN
Bryan Newman, Ph.D. (he/him/his)
Lead Pharmacologist
US Food and Drug Administration
Silver Spring, Maryland, United States - JP
Janny Pineiro-Llanes, Ph.D.
Post Doctoral Associate
University of Florida
Orlando, Florida, United States - BE
Bassma Eltanameli, MS
Graduate Assistant
University of Florida
Orlando, Florida, United States 
Rodrigo Cristofoletti, Ph.D. (he/him/his)
Assistant Professor
University of Florida
Orlando, Florida, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: The Pulmonary Compartmental Absorption and Transit (PCAT™) model within GastroPlus® version 9.8.3 was used to build the lung PBPK models. Two versions of the PBPK model were used: (1) The legacy PCAT model which utilizes only mucus and single tissue layers for each compartment including nose, extra-thoracic, thoracic, bronchioles, and alveoli, and (2) PCAT-2 (Figure 1) which enhances the complexity of each lung tissue compartment by adding separate diffusion sublayers for epithelium, lamina propria, smooth muscle, and endothelium. To parameterize lung deposition and API dissolution in the PCAT model, a combination of in vivo and/or in vitro deposition using various mouth/throat models and Next Generation Impactor data, as well as in silico and in vitro predictions of dissolution, were used [1,2,3]. To validate the IVIVE, the prediction ability of three in vitro cell-based permeability systems (Calu-3, NCI-H441, and MucilAir) were compared for the tobramycin model. For fluticasone propionate, only the in vitro permeability obtained using the MucilAir experiment was tested.
Results: Tobramycin PK predictions using both the legacy PCAT and PCAT-2 with the three cell-based permeability assays are shown in Figure 2. The legacy model does not accurately predict the plasma concentration-time profile due to the assumption of a perfusion-limited systemic uptake from lung tissue into the systemic circulation. Conversely, PCAT-2 doesn’t include this assumption and provides a better prediction of Cmax and AUC when the IVIVE is done with the Calu-3 cell line data. Yet, tmax is not well predicted by this cell line. These subtle differences may be related to the scaling of in vitro effective permeability (Peff) which relies on tissue thickness. For fluticasone PK predictions, the MucilAir experiments could accurately describe the observed data using both the legacy PCAT and PCAT-2 models, as shown in Figure 3. The legacy model had absolute average Cmax, tmax, and AUCinf prediction errors of 18.4%, 350.4%, and 15.06% across the three formulations tested, while the PCAT-2 had absolute average Cmax, tmax, and AUCinf prediction errors of 13.2%, 85.42%, and 27.36%. The models perform comparably but the PCAT-2 produces better absorption dynamics with much lower tmax prediction errors. As fluticasone is a lipophilic API, the assumption of perfusion-limited systemic uptake is accurate in this case whereas it was not for tobramycin.
Conclusion: The PCAT-2 model with an enhanced description of lung physiology provides an improved IVIVE of pulmonary exposure in both case studies. Additional compounds will be evaluated in future work to cement these findings and confirm the enhancement of the mechanistic model. In addition, best practices for the type of in vitro permeability assays to use for a successful IVIVE will be derived for lung PBPK prediction of OIDP absorption predictions.
References: 1. Newhouse, Michael T., et al. Chest 124.1 (2003): 360-366.
2. Thorsson, Lars, et al., British journal of clinical pharmacology 52.5 (2001): 529-538.
3. Hochhaus, Günther, et al. The AAPS journal 23 (2021): 1-14.
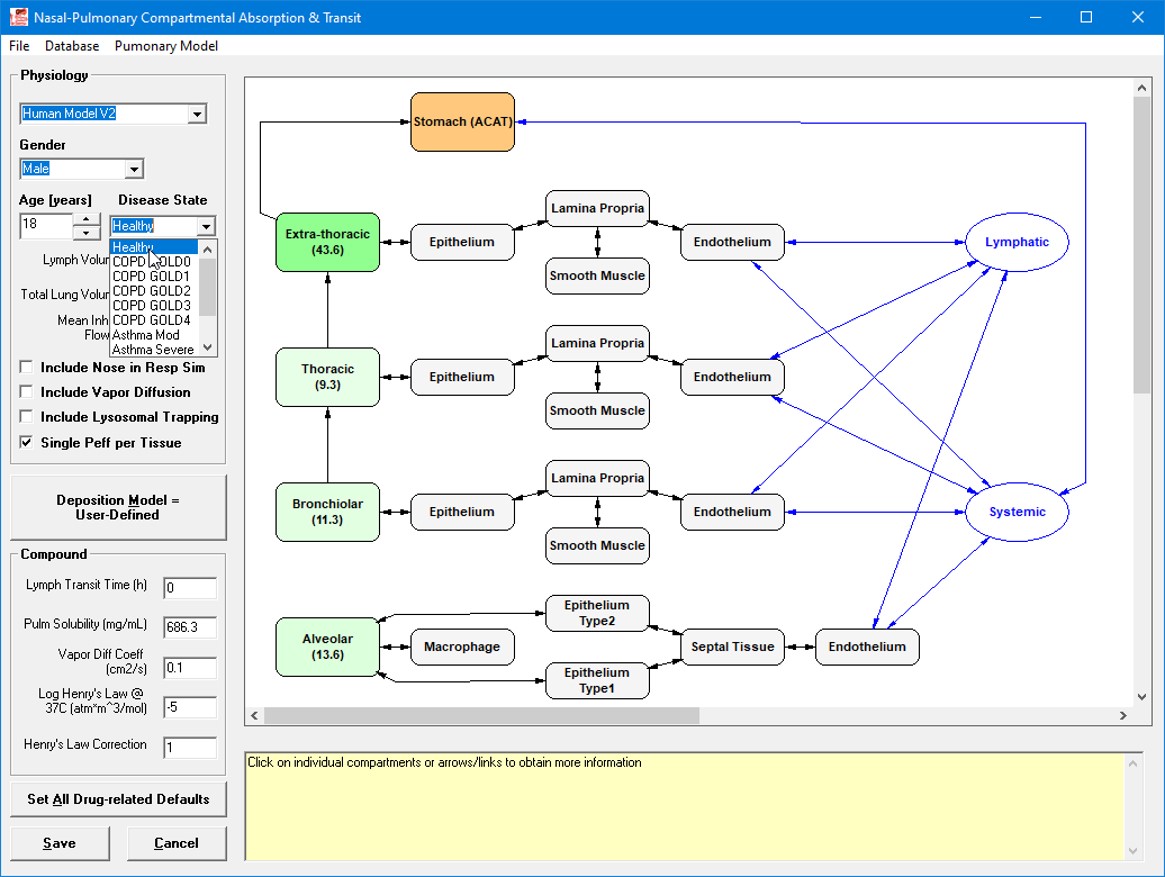 Figure 1: Schematic of Pulmonary Compartmental Absorption and Transit (PCAT™) PCAT-2 with Depiction of Tissue Sublayers
Figure 1: Schematic of Pulmonary Compartmental Absorption and Transit (PCAT™) PCAT-2 with Depiction of Tissue Sublayers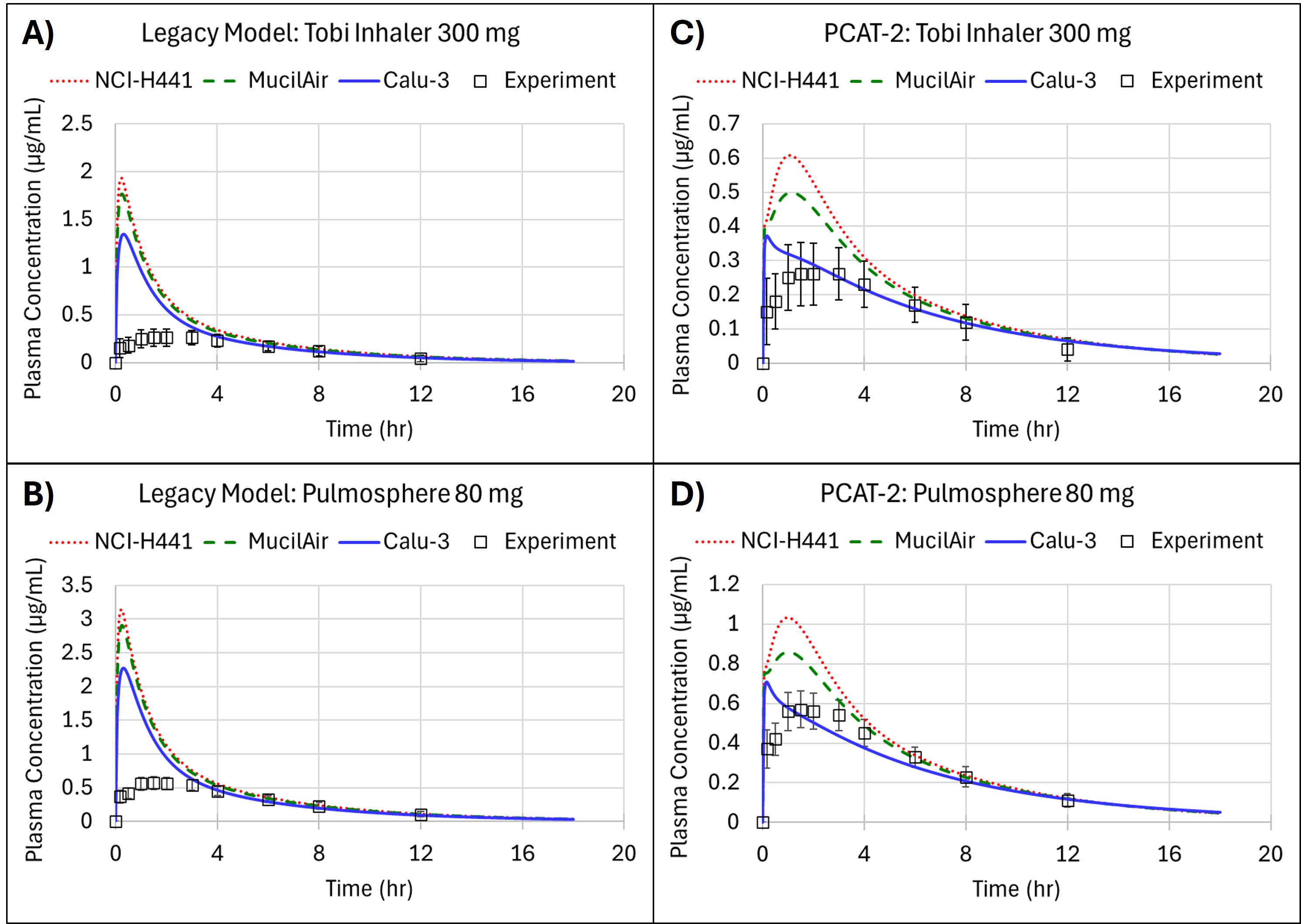 Figure 2: Observed and Predicted Plasma Concentration for Pulmonary PBPK models with In Vitro Permeability Estimates from NCI-H441, MucilAir, and Calu-3 Cell Lines Using Measured Deposition for (A) Legacy Model with Nebulized Tobramycin at 300mg Dose, (B) Legacy Model with Pulmosphere Inhaler at 80mg Dose, (C) PCAT-2 with Tobi Inhaler at 300 mg Dose, and (D) PCAT-2 with Pulmosphere Inhaler at 80 mg Dose.
Figure 2: Observed and Predicted Plasma Concentration for Pulmonary PBPK models with In Vitro Permeability Estimates from NCI-H441, MucilAir, and Calu-3 Cell Lines Using Measured Deposition for (A) Legacy Model with Nebulized Tobramycin at 300mg Dose, (B) Legacy Model with Pulmosphere Inhaler at 80mg Dose, (C) PCAT-2 with Tobi Inhaler at 300 mg Dose, and (D) PCAT-2 with Pulmosphere Inhaler at 80 mg Dose.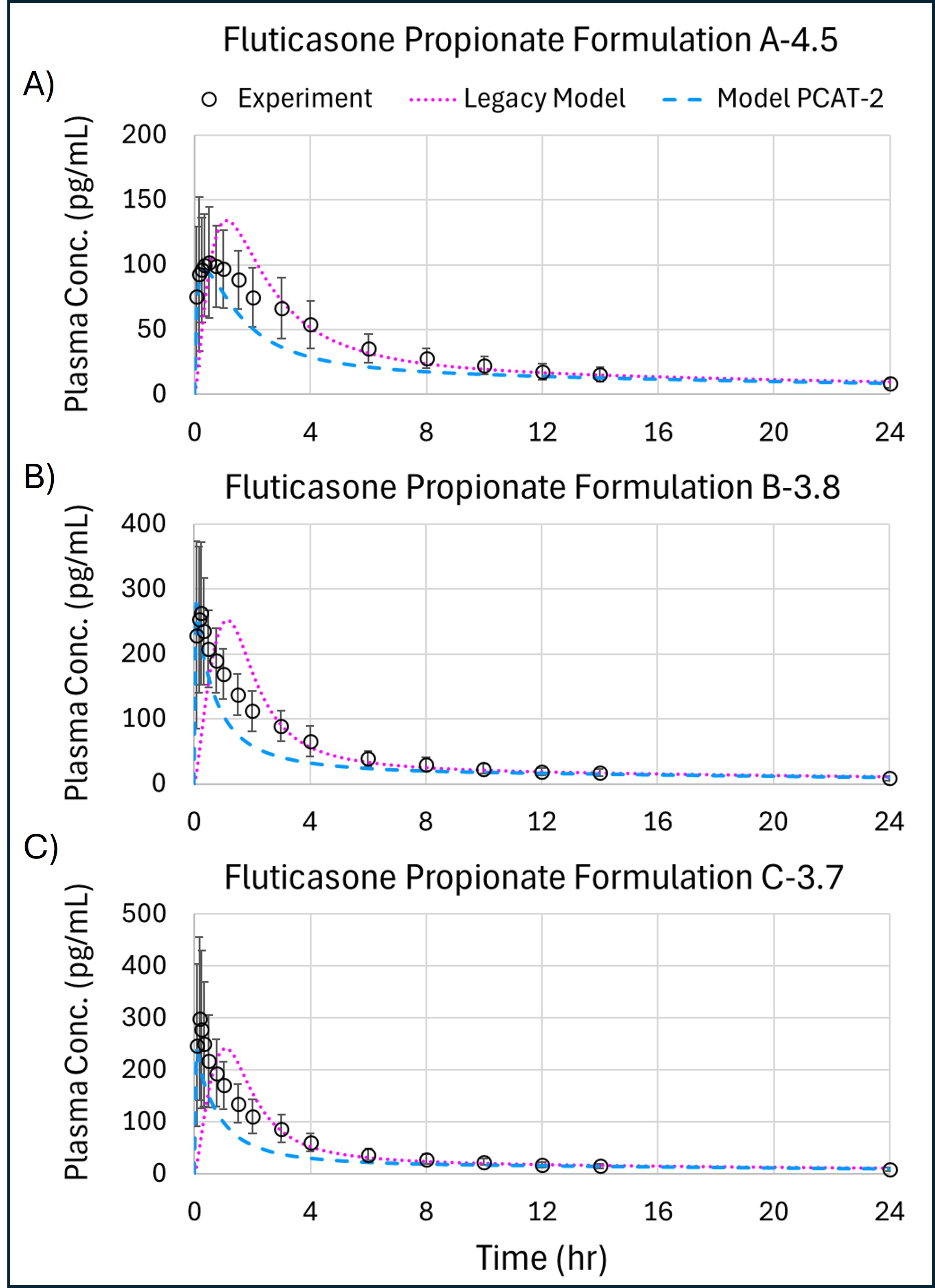 Figure 3: Observed and Predicted Plasma Concentration Profiles for Fluticasone Propionate in Legacy (dotted magenta) and Model Version 2 (dashed teal) for (A) Formulation A-4.5, (B) Formulation B-3.8, and Formulation C-3.7.
Figure 3: Observed and Predicted Plasma Concentration Profiles for Fluticasone Propionate in Legacy (dotted magenta) and Model Version 2 (dashed teal) for (A) Formulation A-4.5, (B) Formulation B-3.8, and Formulation C-3.7.