Formulation and Delivery - Chemical
(T1030-10-59) Unexpected Nucleation of Amorphous Terfenadine at a Much Lower Temperature than the Glass Transition Temperature and Its Impact on Dissolution Behavior and Physical Stability
Tuesday, October 22, 2024
10:30 AM - 11:30 AM MT
- KY
Katsutoshi Yamaguchi, MS (he/him/his)
Scientist
Astellas Pharma Inc.
Tsukuba-shi, Ibaraki, Japan - KY
Katsutoshi Yamaguchi, MS (he/him/his)
Scientist
Astellas Pharma Inc.
Tsukuba-shi, Ibaraki, Japan - YI
Yuya Ishizuka, MS
Scientist
Astellas Pharma Inc.
Tsukuba-shi, Ibaraki, Japan - EY
Etsushi Yoshikawa, MS
Scientist
Astellas Pharma Inc.
Tsukuba-shi, Ibaraki, Japan - TM
Takashi Makishima, MS
Scientist
Astellas Pharma Inc.
Tsukuba-shi, Ibaraki, Japan - RM
Ryo Mizoguchi, Ph.D.
Head of Pharmaceutical Developability
Astellas Pharma Inc.
Tsukuba-shi, Ibaraki, Japan 
Kohsaku Kawakami, Ph.D. (he/him/his)
Group Leader
National Institute for Materials Science
Tsukuba, Ibaraki, Japan
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Amorphization is a powerful approach to address the low solubility issue of pharmaceutical compounds. However, the amorphous form is not physically stable and has the risk of crystallization during storage and upon contact with water. It is commonly understood that amorphous pharmaceutical compounds have sufficient physical stability for a few years as long as they are stored at a temperature at least 50 °C below the glass transition temperature1, 2. Therefore, amorphous drug substances or formulations are sometimes stored under refrigerated or frozen conditions in the pharmaceutical industry. However, an exceptional case has been observed in which an amorphous pharmaceutical compound stored under a sufficiently low temperature showed enhanced crystallization tendency and impeded dissolution performance compared to the same compound stored under ambient temperature due to nucleation at the lower temperature3, 4. We found that amorphous terfenadine exhibited similar behavior. In this study, we report the effect of storage temperature and time on the crystallization tendency, physical stability and supersaturation behavior of amorphous terfenadine, focusing on the nucleation process.
Methods: Amorphous terfenadine was prepared by a melt-quench method using DSC and annealed at different temperatures in the range of -80 °C to 40 °C for up to 40 days. Nucleation of the annealed samples was evaluated by investigating the effect of the annealing on the onset temperature of cold crystallization during the DSC heating and resultant crystal forms. Amorphous terfenadine is generally classified in Class III, which does not crystallize during cooling from the melt at 20 °C/min and reheating at 10 °C/min, according to the crystallization tendency classification system5. Therefore, a slower heating rate of 1 °C/min was used to allow growth of the crystal from the nuclei formed during annealing. Furthermore, the effect of annealing temperature and time on physical stability and supersaturation behavior of amorphous terfenadine were evaluated. The physical stability of annealed samples at 100 °C was evaluated by modulated DSC and the time at which 10% of the amorphous fraction crystallized, t90, was calculated using the modified Avrami equation6. Dissolution profile and supersaturation behavior of the annealed samples in buffered solution were evaluated using µDISS Profiler®, and the time at which concentration started to decrease, tmax, was identified as a parameter to evaluate supersaturation stability.
Results: During heating in DSC, amorphous terfenadine annealed at -20 °C for 1 day showed glass transition, an exothermic crystallization peak, and endothermic melting peaks of form II and form I at ca. 55 °C, 125 °C, 148 °C, and 151 °C, respectively (Figure 1). In contrast, freshly prepared amorphous terfenadine showed no thermal events except for glass transition. We comprehensively investigated the effect of annealing temperature and time on crystallization behavior of amorphous terfenadine. Amorphous terfenadine annealed at -30 °C and -20 °C showed the lowest onset temperature of cold crystallization which decreased with increasing annealing time (Figure 2(a)). In contrast, crystallization and melting peaks were not observed for samples annealed at -80 °C. Furthermore, the ratio of heat of fusion of form II to form I of annealed samples showed the largest value for the samples annealed at -30 °C and -20 °C, and increased with increasing annealing time (Figure 2(b)). These results indicate that nucleation of form II preferentially occurred at -30 °C and -20 °C, which are lower than the glass transition temperature by more than 50 °C. Next, we evaluated the effect of nucleation on physical stability and supersaturation behavior. Isothermal crystallization at 100 °C of amorphous terfenadine annealed at -20 °C showed a faster crystallization than those annealed at 25 °C and 40 °C (Figure 3). These annealed samples were confirmed to crystallize into form II based on the melting behavior. However, annealing temperature did not affect tmax values in the dissolution study. Powder X-ray diffraction patterns of residues in the dissolution study differed from those of form I and form II, which was likely the reason for the lack of impact of the crystal nuclei of form II formed during annealing.
Conclusion: The effect of annealing temperature and time on the crystallization behavior of amorphous terfenadine was evaluated in this study. Nucleation of form II preferentially occurred at -30 °C and -20 °C, which was lower than the glass transition temperature by more than 50 °C. The physical stability study of amorphous terfenadine annealed at -20 °C provided the smallest t90 value. However, supersaturation behavior was not influenced by annealing temperature, since the crystal form appeared during the dissolution study differed from that of the crystal nuclei formed during annealing. These results emphasize the importance of the rational setting of storage temperature for amorphous materials based on an understanding of nucleation behavior.
References: 1. Hancock, B. C.; Shamblin, S. L.; Zografi, G., Molecular mobility of amorphous pharmaceutical solids below their glass transition temperatures. Pharm. Res. 1995, 12 (6), 799-806.
2. Yu, L., Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001, 48 (1), 27-42.
3. Kawakami, K., Nucleation and crystallization of celecoxib glass: Impact of experience of low temperature on physical stability. Thermochim. Acta 2019, 671, 43-47.
4. Song, J.; Kawakami, K., Nucleation During Storage Impeded Supersaturation in the Dissolution Process of Amorphous Celecoxib. Mol. Pharmaceutics 2023, 20 (8), 4050-4057.
5. Baird, J. A.; Van Eerdenbrugh, B.; Taylor, L. S., A Classification System to Assess the Crystallization Tendency of Organic Molecules from Undercooled Melts. J. Pharm. Sci. 2010, 99 (9), 3787-3806.
6. Yamaguchi, K.; Mizoguchi, R.; Kawakami, K.; Miyazaki, T., Influence of the crystallization tendencies of pharmaceutical glasses on the applicability of the Adam-Gibbs-Vogel and Vogel-Tammann-Fulcher equations in the prediction of their long-term physical stability. Int. J. Pharm. 2022, 626, 122158.
Acknowledgements: This study was conducted as part of the Materials Open Platform for Pharmaceutical Science, led by the National Institute for Materials Science.
This work was funded by the Materials Open Platform for Pharmaceutical Science and each author’s affiliation.
The authors declare no conflict of interest.
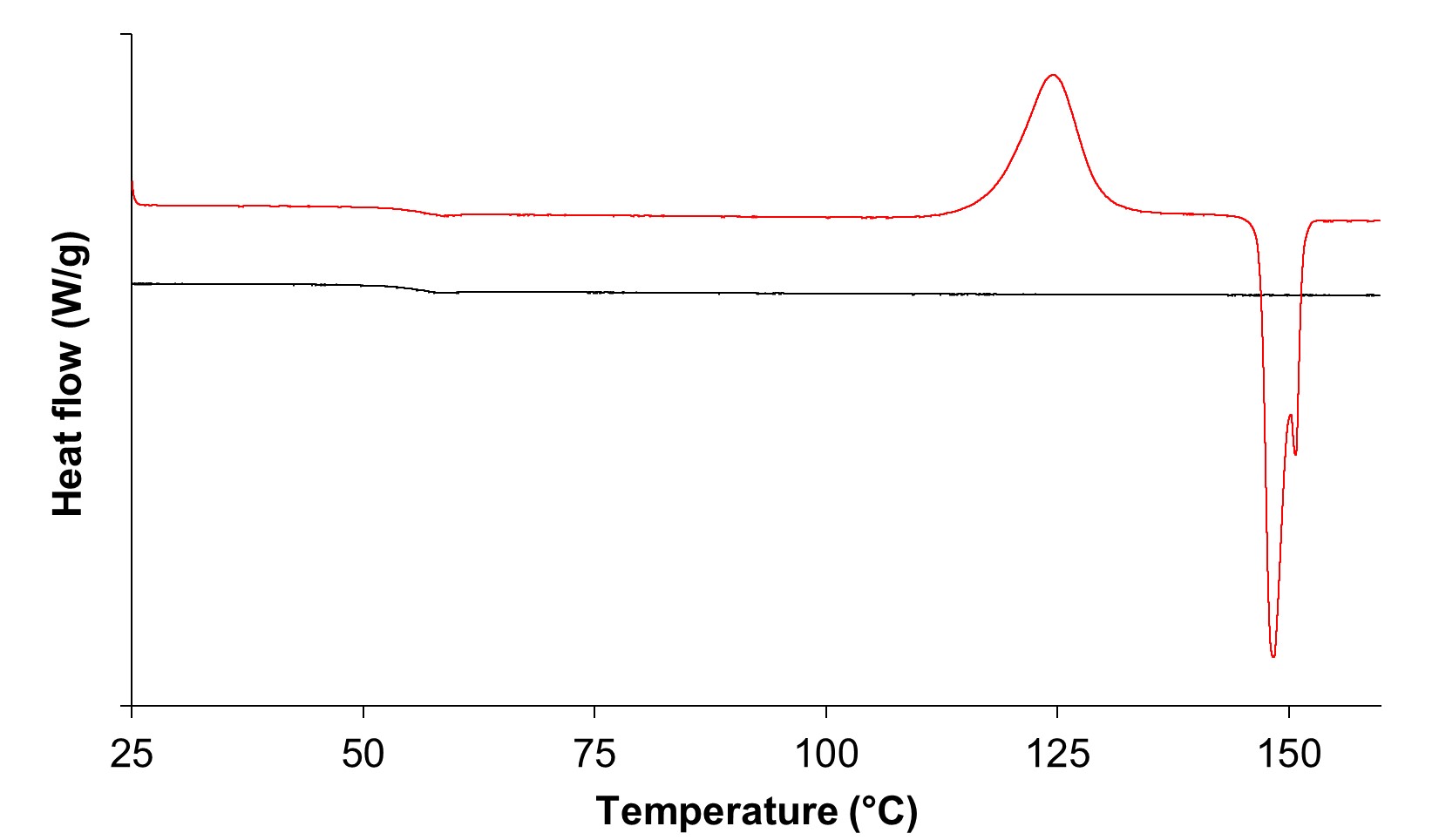 Figure 1. DSC thermograms of amorphous terfenadine immediately after preparation (black) and after annealing at -20 °C for 1 day (red).
Figure 1. DSC thermograms of amorphous terfenadine immediately after preparation (black) and after annealing at -20 °C for 1 day (red).
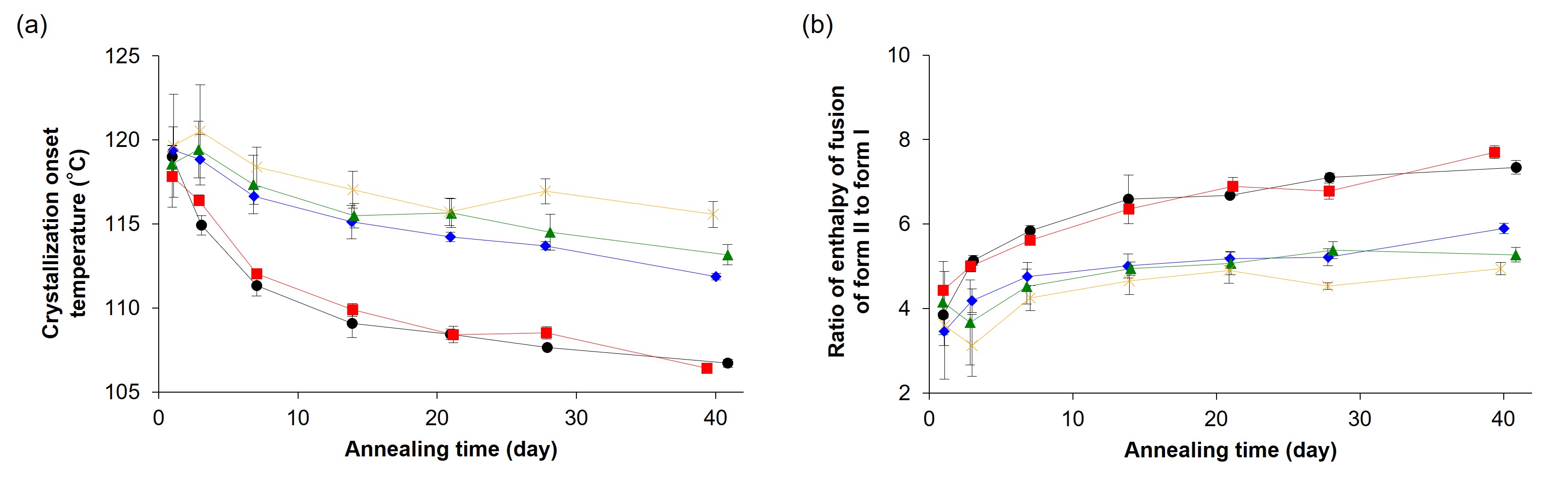 Figure 2. Effect of annealing temperature and time on (a) crystallization onset temperature and (b) ratio of enthalpy of fusion of form II to form I. Symbols indicate the following: -30 °C (black circle), -20 °C (red square), 5 °C (blue diamond), 25 °C (green triangle) and 40 °C (yellow cross).
Figure 2. Effect of annealing temperature and time on (a) crystallization onset temperature and (b) ratio of enthalpy of fusion of form II to form I. Symbols indicate the following: -30 °C (black circle), -20 °C (red square), 5 °C (blue diamond), 25 °C (green triangle) and 40 °C (yellow cross).
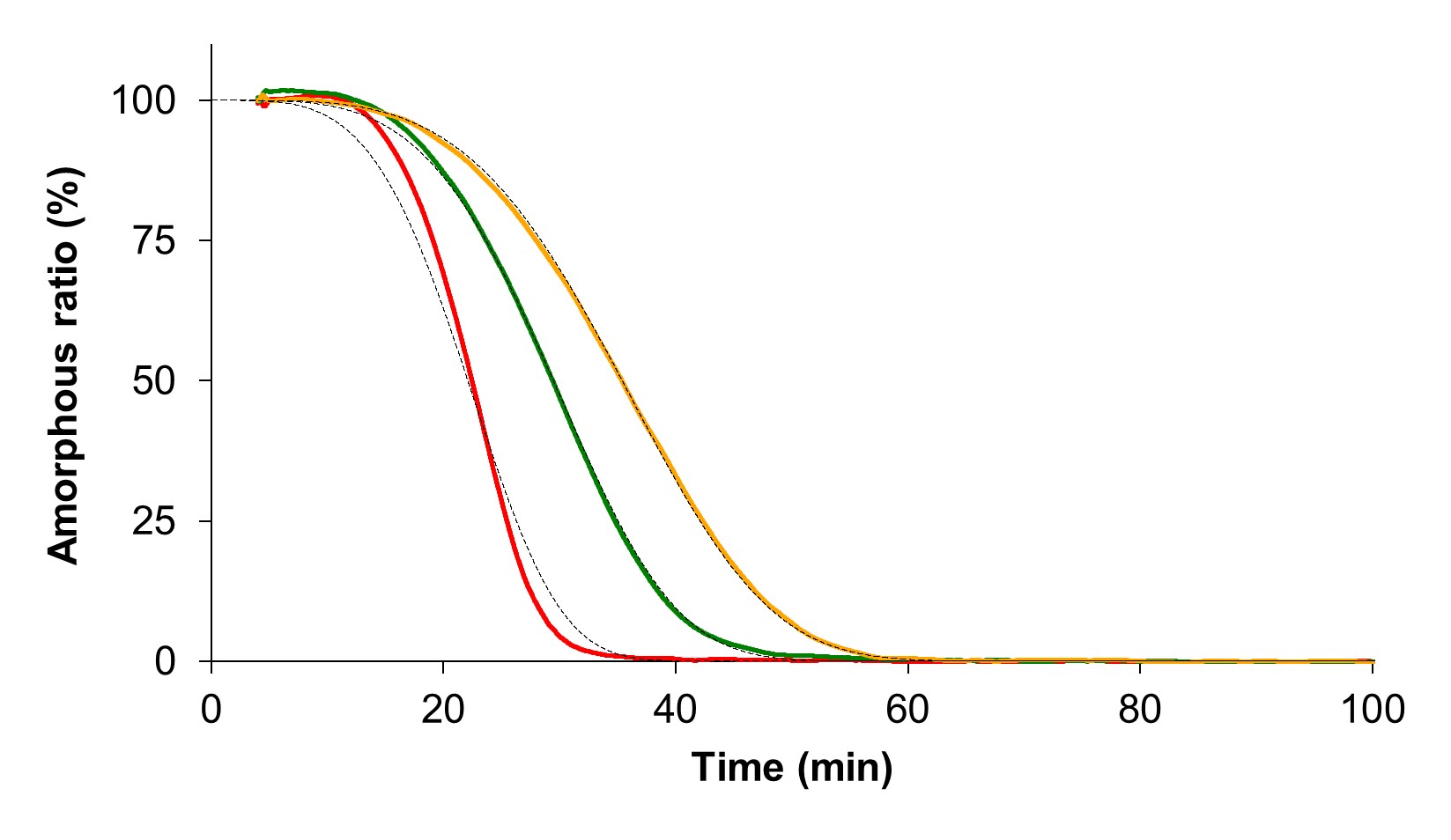 Figure 3. Decrease in the amorphous fraction of terfenadine during isothermal holding at 100 °C. The samples were annealed at -20 °C (red), 25 °C (green) and 40 °C (yellow) for 40 days before being subjected to 100 °C. The data were fitted by the modified Avrami equation (dotted line).
Figure 3. Decrease in the amorphous fraction of terfenadine during isothermal holding at 100 °C. The samples were annealed at -20 °C (red), 25 °C (green) and 40 °C (yellow) for 40 days before being subjected to 100 °C. The data were fitted by the modified Avrami equation (dotted line).
Methods: Amorphous terfenadine was prepared by a melt-quench method using DSC and annealed at different temperatures in the range of -80 °C to 40 °C for up to 40 days. Nucleation of the annealed samples was evaluated by investigating the effect of the annealing on the onset temperature of cold crystallization during the DSC heating and resultant crystal forms. Amorphous terfenadine is generally classified in Class III, which does not crystallize during cooling from the melt at 20 °C/min and reheating at 10 °C/min, according to the crystallization tendency classification system5. Therefore, a slower heating rate of 1 °C/min was used to allow growth of the crystal from the nuclei formed during annealing. Furthermore, the effect of annealing temperature and time on physical stability and supersaturation behavior of amorphous terfenadine were evaluated. The physical stability of annealed samples at 100 °C was evaluated by modulated DSC and the time at which 10% of the amorphous fraction crystallized, t90, was calculated using the modified Avrami equation6. Dissolution profile and supersaturation behavior of the annealed samples in buffered solution were evaluated using µDISS Profiler®, and the time at which concentration started to decrease, tmax, was identified as a parameter to evaluate supersaturation stability.
Results: During heating in DSC, amorphous terfenadine annealed at -20 °C for 1 day showed glass transition, an exothermic crystallization peak, and endothermic melting peaks of form II and form I at ca. 55 °C, 125 °C, 148 °C, and 151 °C, respectively (Figure 1). In contrast, freshly prepared amorphous terfenadine showed no thermal events except for glass transition. We comprehensively investigated the effect of annealing temperature and time on crystallization behavior of amorphous terfenadine. Amorphous terfenadine annealed at -30 °C and -20 °C showed the lowest onset temperature of cold crystallization which decreased with increasing annealing time (Figure 2(a)). In contrast, crystallization and melting peaks were not observed for samples annealed at -80 °C. Furthermore, the ratio of heat of fusion of form II to form I of annealed samples showed the largest value for the samples annealed at -30 °C and -20 °C, and increased with increasing annealing time (Figure 2(b)). These results indicate that nucleation of form II preferentially occurred at -30 °C and -20 °C, which are lower than the glass transition temperature by more than 50 °C. Next, we evaluated the effect of nucleation on physical stability and supersaturation behavior. Isothermal crystallization at 100 °C of amorphous terfenadine annealed at -20 °C showed a faster crystallization than those annealed at 25 °C and 40 °C (Figure 3). These annealed samples were confirmed to crystallize into form II based on the melting behavior. However, annealing temperature did not affect tmax values in the dissolution study. Powder X-ray diffraction patterns of residues in the dissolution study differed from those of form I and form II, which was likely the reason for the lack of impact of the crystal nuclei of form II formed during annealing.
Conclusion: The effect of annealing temperature and time on the crystallization behavior of amorphous terfenadine was evaluated in this study. Nucleation of form II preferentially occurred at -30 °C and -20 °C, which was lower than the glass transition temperature by more than 50 °C. The physical stability study of amorphous terfenadine annealed at -20 °C provided the smallest t90 value. However, supersaturation behavior was not influenced by annealing temperature, since the crystal form appeared during the dissolution study differed from that of the crystal nuclei formed during annealing. These results emphasize the importance of the rational setting of storage temperature for amorphous materials based on an understanding of nucleation behavior.
References: 1. Hancock, B. C.; Shamblin, S. L.; Zografi, G., Molecular mobility of amorphous pharmaceutical solids below their glass transition temperatures. Pharm. Res. 1995, 12 (6), 799-806.
2. Yu, L., Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001, 48 (1), 27-42.
3. Kawakami, K., Nucleation and crystallization of celecoxib glass: Impact of experience of low temperature on physical stability. Thermochim. Acta 2019, 671, 43-47.
4. Song, J.; Kawakami, K., Nucleation During Storage Impeded Supersaturation in the Dissolution Process of Amorphous Celecoxib. Mol. Pharmaceutics 2023, 20 (8), 4050-4057.
5. Baird, J. A.; Van Eerdenbrugh, B.; Taylor, L. S., A Classification System to Assess the Crystallization Tendency of Organic Molecules from Undercooled Melts. J. Pharm. Sci. 2010, 99 (9), 3787-3806.
6. Yamaguchi, K.; Mizoguchi, R.; Kawakami, K.; Miyazaki, T., Influence of the crystallization tendencies of pharmaceutical glasses on the applicability of the Adam-Gibbs-Vogel and Vogel-Tammann-Fulcher equations in the prediction of their long-term physical stability. Int. J. Pharm. 2022, 626, 122158.
Acknowledgements: This study was conducted as part of the Materials Open Platform for Pharmaceutical Science, led by the National Institute for Materials Science.
This work was funded by the Materials Open Platform for Pharmaceutical Science and each author’s affiliation.
The authors declare no conflict of interest.
 Figure 1. DSC thermograms of amorphous terfenadine immediately after preparation (black) and after annealing at -20 °C for 1 day (red).
Figure 1. DSC thermograms of amorphous terfenadine immediately after preparation (black) and after annealing at -20 °C for 1 day (red). Figure 2. Effect of annealing temperature and time on (a) crystallization onset temperature and (b) ratio of enthalpy of fusion of form II to form I. Symbols indicate the following: -30 °C (black circle), -20 °C (red square), 5 °C (blue diamond), 25 °C (green triangle) and 40 °C (yellow cross).
Figure 2. Effect of annealing temperature and time on (a) crystallization onset temperature and (b) ratio of enthalpy of fusion of form II to form I. Symbols indicate the following: -30 °C (black circle), -20 °C (red square), 5 °C (blue diamond), 25 °C (green triangle) and 40 °C (yellow cross). Figure 3. Decrease in the amorphous fraction of terfenadine during isothermal holding at 100 °C. The samples were annealed at -20 °C (red), 25 °C (green) and 40 °C (yellow) for 40 days before being subjected to 100 °C. The data were fitted by the modified Avrami equation (dotted line).
Figure 3. Decrease in the amorphous fraction of terfenadine during isothermal holding at 100 °C. The samples were annealed at -20 °C (red), 25 °C (green) and 40 °C (yellow) for 40 days before being subjected to 100 °C. The data were fitted by the modified Avrami equation (dotted line).