Formulation and Delivery - Chemical
(T1130-07-39) Development of Novel Combination Therapy containing Lobeglitazone and Its Clinical Studies in Patients with Poorly Controlled Type 2 Diabetes
Tuesday, October 22, 2024
11:30 AM - 12:30 PM MT
- TK
Taehyung Kim, MS
Senior Research Scientist III
Chong Kun Dang Pharmaceutical Corp.
Yongin, Kyonggi-do, Republic of Korea - MK
MinYoung Kim, MS
Senior Research Scientist I
Chong Kun Dang Pharmaceutical Corp.
Yongin, Kyonggi-do, Republic of Korea - SH
SungKyun Han, Ph.D.
Principal Research Scientist I
Chong Kun Dang Pharmaceutical Corp.
Yongin, Kyonggi-do, Republic of Korea
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Diabetes is a chronic disease characterized by impaired blood sugar control, which leads to various complications and highlighting the necessity of effective glycemic management through appropriate treatment. [1] The majority of diabetes patients have type 2 diabetes, primarily controlling their blood sugar with metformin monotherapy. [2] However, due to insufficient glycemic management with metformin alone, there is an increasing demand for combination therapies that includes two or three drugs. [3] Lobeglitazone (CKD-501) is a thiazolidinedione known as a peroxisome proliferator-activated receptors gamma (PPARγ) agonist. This study aims to develop CKD-396 formulation, a fixed-combination drug of lobeglitazone and the well-known DPP4 inhibitor sitagliptin, as well as CKD-393, a triple fixed-combination drug that includes lobeglitazone, sitagliptin, and metformin, to enhance patient convenience and compliance.
Methods: (Preparation of Drug Products) CKD-396 was developed as an immediate-release (IR) tablet combining lobeglitazone and sitagliptin in a single matrix. Meanwhile, CKD-393 was developed as a bi-layer tablet consisting of an IR layer with lobeglitazone and sitagliptin and an extended-release (XR) layer with metformin. (In Vitro Dissolution & In Vivo Preclinical PK) Two formulations of CKD-396 were investigated for in vitro dissolution and in vivo preclinical pharmacokinetic(PK) studies, using Duvie® and Januvia® tablets as reference drugs. In vitro dissolution was performed using a CE7 (SOTAX®) instrument, employing a USP dissolution apparatus 4 (Flow Through Cell, FTC), at a flow rate of 4.0 mL/min in artificial gastric fluid (pH 1.2 buffer). In vivo preclinical PK study was evaluated in 12 beagle dogs, arranged in a 4ⅹ4 crossover design (3 dogs per group), under fasting conditions with a one-week washout period between treatments. (Phase 1 Clinical Trials) Following the investigation of CKD-396 formulation, which exhibited in vitro dissolution and in vivo PK profiles closely matching those of the reference drugs, CKD-393 was subsequently developed by incorporating an extended-release metformin layer. Two Phase 1 bioequivalence(BE) studies were conducted for CKD-396 and CKD-393 with 26 subjects in a 2ⅹ2 crossover design (13 subjects per group) under both fasting and high-fat diet conditions. The reference drugs for CKD-396 were Duvie® and Januvia®, while for CKD-393, the reference drugs were Duvie®, Januvia®, and Glucophage® XR. (Phase 3 Clinical Trial) This multicenter, randomized, double-blind, parallel, placebo-controlled, phase 3 study aimed to evaluate the efficacy of adding lobeglitazone to metformin and sitagliptin therapy in 231 patients with type 2 diabetes mullitus(T2DM) who were inadequately controlled with metformin and sitagliptin alone. Over a period of 24 weeks, patients were monitored, with HbA1c levels serving as the primary efficacy endpoint. Statistical analysis was conducted using ANCOVA to assess the significance of the results.
Results: (In Vitro Dissolution and Preclinical PK) In the in vitro dissolution tests, CKD-396 formulation I demonstrated dissolution rates comparable to or faster than the reference drugs, while formulation II exhibited slower rates. In the in vivo preclinical PK study, formulation I showed BE with the reference drugs, whereas formulation II showed lower Cmax and AUC values compared to the reference drug. (Phase 1 Clinical Trials) CKD-396 formulation I was found to be bioequivalent to the reference drugs. Additionally, CKD-393, a bi-layer tablet with an IR layer formulation based on the formulation I of CKD-396, also demonstrated BE with each reference drug. (Phase 3 Clinical Trial) After 24 weeks of treatment, the test group represented a statistically significant reduction in HbA1c (-1.00%, p< 0.0001) compared to the placebo group, which showed a slight increase in HbA1c (+0.02%, p=0.7944). The difference between these two groups was statistically significant (p < 0.0001), confirming the superior efficacy of lobeglitazone in reducing HbA1c levels.
Conclusion: We successfully developed the dual combination drug CKD-396 (Duvie® S) containing lobeglitazone and sitagliptin, and the triple combination drug CKD-393 (Duvimet® S), which includes extended-release metformin. Clinical trials demonstrated that these combination therapies represent PK similar to those of existing monotherapies and are effective in reducing blood glucose levels in patients inadequately controlled with only sitagliptin and metformin. These findings suggest that lobeglitazone combination therapies provide an improved treatment option for T2DM patients, enhancing compliance and glycemic control. Both CKD-396 and CKD-393 were approved by the Korean Ministry of Food and Drug Safety and launched in 2023.
References: [1] Gerstein HC et al. N Engl J Med 2008;358:2545–59.
[2] Wu D et al. Diabetes Obes Metab 2014;16:30–7.
[3] Lim S et al. BMJ Open Diab Res Care 2020;8:e000807.
.jpg) Figure 1. In vitro release profiles of (A) lobeglitazone and (B) sitagliptin using a flow through cell dissolution tester in a pH 1.2 buffer solution, and average plasma concentration vs. time profiles of (A) lobeglitazone and (B) sitagliptin following oral administration of phase 1 reference drugs and CKD-396 test formulations.
Figure 1. In vitro release profiles of (A) lobeglitazone and (B) sitagliptin using a flow through cell dissolution tester in a pH 1.2 buffer solution, and average plasma concentration vs. time profiles of (A) lobeglitazone and (B) sitagliptin following oral administration of phase 1 reference drugs and CKD-396 test formulations.
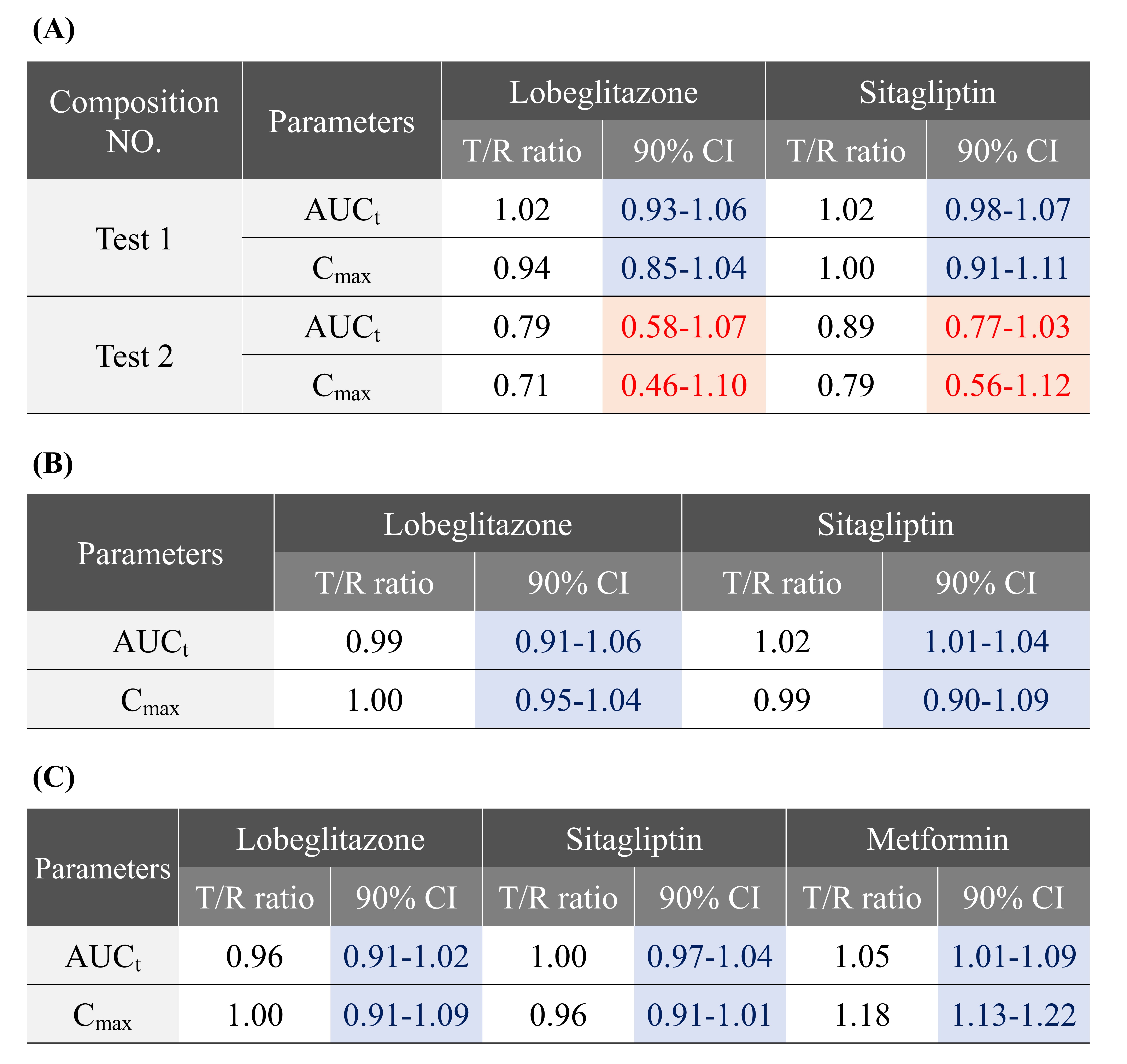 Table 1. Geometric mean test/reference ratios and 90% confidence intervals for log-transformed pharmacokinetic parameters (Cmax and AUCt) between the test and reference drugs. (A) Results of preclinical pharmacokinetic study of CKD-396 test 1 and 2 compositions, (B) Results of phase 1 bioequivalence study of CKD-396, and (C) Results of phase 1 bioequivalence study of CKD-393.
Table 1. Geometric mean test/reference ratios and 90% confidence intervals for log-transformed pharmacokinetic parameters (Cmax and AUCt) between the test and reference drugs. (A) Results of preclinical pharmacokinetic study of CKD-396 test 1 and 2 compositions, (B) Results of phase 1 bioequivalence study of CKD-396, and (C) Results of phase 1 bioequivalence study of CKD-393.
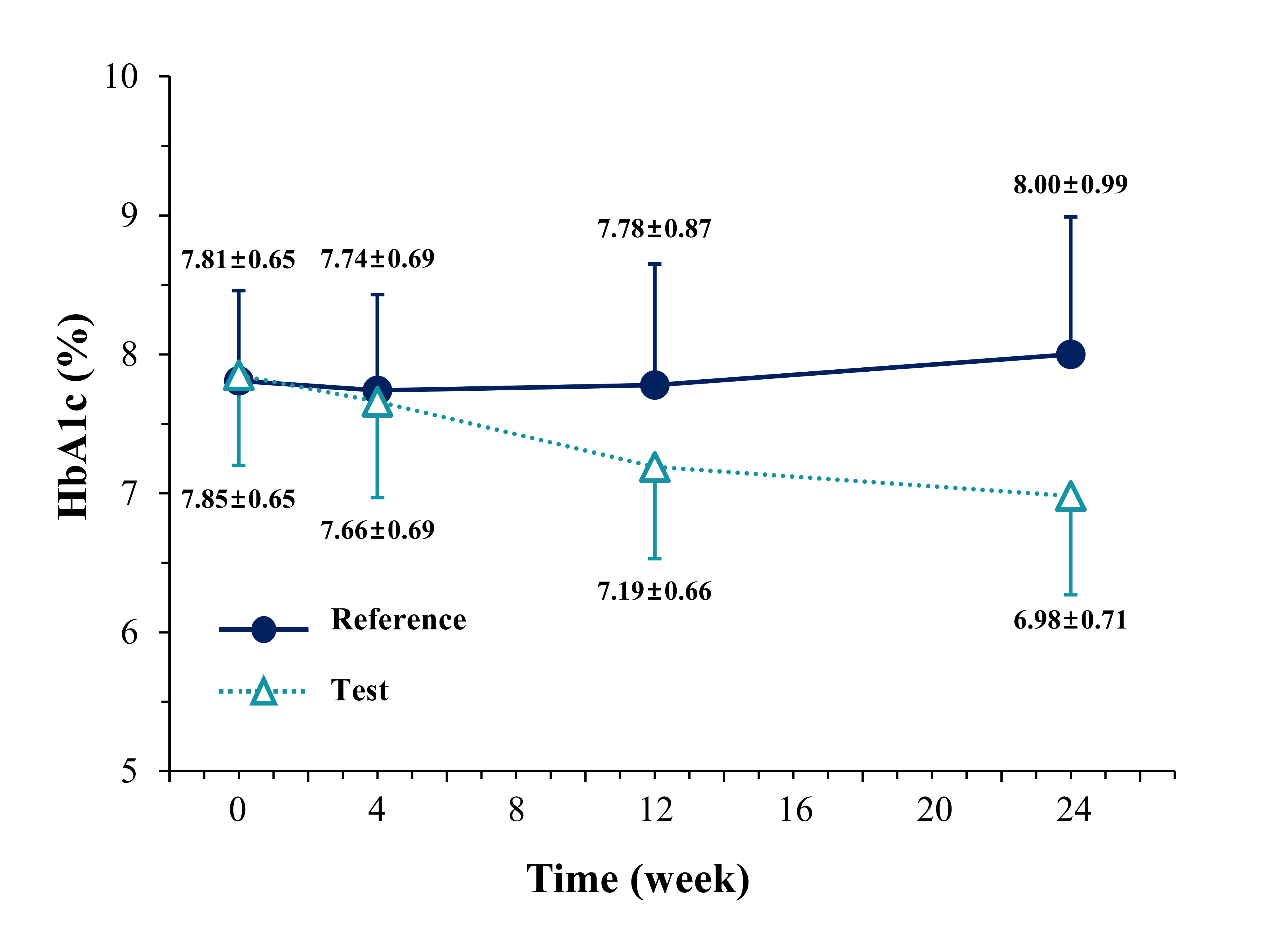 Figure 2. Temporal changes in HbA1c levels (%) at baseline and 4, 12, and 24 weeks of follow-up in T2DM patients (n=231) insufficiently controlled with metformin and sitagliptin. Both groups received metformin hydrochloride (≥1,000 mg) and sitagliptin 100 mg. The control group received a placebo for lobeglitazone sulfate, while the test group received lobeglitazone sulfate 0.5 mg.
Figure 2. Temporal changes in HbA1c levels (%) at baseline and 4, 12, and 24 weeks of follow-up in T2DM patients (n=231) insufficiently controlled with metformin and sitagliptin. Both groups received metformin hydrochloride (≥1,000 mg) and sitagliptin 100 mg. The control group received a placebo for lobeglitazone sulfate, while the test group received lobeglitazone sulfate 0.5 mg.
Methods: (Preparation of Drug Products) CKD-396 was developed as an immediate-release (IR) tablet combining lobeglitazone and sitagliptin in a single matrix. Meanwhile, CKD-393 was developed as a bi-layer tablet consisting of an IR layer with lobeglitazone and sitagliptin and an extended-release (XR) layer with metformin. (In Vitro Dissolution & In Vivo Preclinical PK) Two formulations of CKD-396 were investigated for in vitro dissolution and in vivo preclinical pharmacokinetic(PK) studies, using Duvie® and Januvia® tablets as reference drugs. In vitro dissolution was performed using a CE7 (SOTAX®) instrument, employing a USP dissolution apparatus 4 (Flow Through Cell, FTC), at a flow rate of 4.0 mL/min in artificial gastric fluid (pH 1.2 buffer). In vivo preclinical PK study was evaluated in 12 beagle dogs, arranged in a 4ⅹ4 crossover design (3 dogs per group), under fasting conditions with a one-week washout period between treatments. (Phase 1 Clinical Trials) Following the investigation of CKD-396 formulation, which exhibited in vitro dissolution and in vivo PK profiles closely matching those of the reference drugs, CKD-393 was subsequently developed by incorporating an extended-release metformin layer. Two Phase 1 bioequivalence(BE) studies were conducted for CKD-396 and CKD-393 with 26 subjects in a 2ⅹ2 crossover design (13 subjects per group) under both fasting and high-fat diet conditions. The reference drugs for CKD-396 were Duvie® and Januvia®, while for CKD-393, the reference drugs were Duvie®, Januvia®, and Glucophage® XR. (Phase 3 Clinical Trial) This multicenter, randomized, double-blind, parallel, placebo-controlled, phase 3 study aimed to evaluate the efficacy of adding lobeglitazone to metformin and sitagliptin therapy in 231 patients with type 2 diabetes mullitus(T2DM) who were inadequately controlled with metformin and sitagliptin alone. Over a period of 24 weeks, patients were monitored, with HbA1c levels serving as the primary efficacy endpoint. Statistical analysis was conducted using ANCOVA to assess the significance of the results.
Results: (In Vitro Dissolution and Preclinical PK) In the in vitro dissolution tests, CKD-396 formulation I demonstrated dissolution rates comparable to or faster than the reference drugs, while formulation II exhibited slower rates. In the in vivo preclinical PK study, formulation I showed BE with the reference drugs, whereas formulation II showed lower Cmax and AUC values compared to the reference drug. (Phase 1 Clinical Trials) CKD-396 formulation I was found to be bioequivalent to the reference drugs. Additionally, CKD-393, a bi-layer tablet with an IR layer formulation based on the formulation I of CKD-396, also demonstrated BE with each reference drug. (Phase 3 Clinical Trial) After 24 weeks of treatment, the test group represented a statistically significant reduction in HbA1c (-1.00%, p< 0.0001) compared to the placebo group, which showed a slight increase in HbA1c (+0.02%, p=0.7944). The difference between these two groups was statistically significant (p < 0.0001), confirming the superior efficacy of lobeglitazone in reducing HbA1c levels.
Conclusion: We successfully developed the dual combination drug CKD-396 (Duvie® S) containing lobeglitazone and sitagliptin, and the triple combination drug CKD-393 (Duvimet® S), which includes extended-release metformin. Clinical trials demonstrated that these combination therapies represent PK similar to those of existing monotherapies and are effective in reducing blood glucose levels in patients inadequately controlled with only sitagliptin and metformin. These findings suggest that lobeglitazone combination therapies provide an improved treatment option for T2DM patients, enhancing compliance and glycemic control. Both CKD-396 and CKD-393 were approved by the Korean Ministry of Food and Drug Safety and launched in 2023.
References: [1] Gerstein HC et al. N Engl J Med 2008;358:2545–59.
[2] Wu D et al. Diabetes Obes Metab 2014;16:30–7.
[3] Lim S et al. BMJ Open Diab Res Care 2020;8:e000807.
.jpg) Figure 1. In vitro release profiles of (A) lobeglitazone and (B) sitagliptin using a flow through cell dissolution tester in a pH 1.2 buffer solution, and average plasma concentration vs. time profiles of (A) lobeglitazone and (B) sitagliptin following oral administration of phase 1 reference drugs and CKD-396 test formulations.
Figure 1. In vitro release profiles of (A) lobeglitazone and (B) sitagliptin using a flow through cell dissolution tester in a pH 1.2 buffer solution, and average plasma concentration vs. time profiles of (A) lobeglitazone and (B) sitagliptin following oral administration of phase 1 reference drugs and CKD-396 test formulations. Table 1. Geometric mean test/reference ratios and 90% confidence intervals for log-transformed pharmacokinetic parameters (Cmax and AUCt) between the test and reference drugs. (A) Results of preclinical pharmacokinetic study of CKD-396 test 1 and 2 compositions, (B) Results of phase 1 bioequivalence study of CKD-396, and (C) Results of phase 1 bioequivalence study of CKD-393.
Table 1. Geometric mean test/reference ratios and 90% confidence intervals for log-transformed pharmacokinetic parameters (Cmax and AUCt) between the test and reference drugs. (A) Results of preclinical pharmacokinetic study of CKD-396 test 1 and 2 compositions, (B) Results of phase 1 bioequivalence study of CKD-396, and (C) Results of phase 1 bioequivalence study of CKD-393. Figure 2. Temporal changes in HbA1c levels (%) at baseline and 4, 12, and 24 weeks of follow-up in T2DM patients (n=231) insufficiently controlled with metformin and sitagliptin. Both groups received metformin hydrochloride (≥1,000 mg) and sitagliptin 100 mg. The control group received a placebo for lobeglitazone sulfate, while the test group received lobeglitazone sulfate 0.5 mg.
Figure 2. Temporal changes in HbA1c levels (%) at baseline and 4, 12, and 24 weeks of follow-up in T2DM patients (n=231) insufficiently controlled with metformin and sitagliptin. Both groups received metformin hydrochloride (≥1,000 mg) and sitagliptin 100 mg. The control group received a placebo for lobeglitazone sulfate, while the test group received lobeglitazone sulfate 0.5 mg.