Formulation and Delivery - Chemical
(T1130-07-38) Deformable Polydopamine Nanocapsules for siRNA Delivery to Solid Tumors
Tuesday, October 22, 2024
11:30 AM - 12:30 PM MT
.jpg)
Hytham H. Gadalla, MS (he/him/his)
Graduate student
Purdue University
West Lafayette, Indiana, United States.jpg)
Hytham H. Gadalla, MS (he/him/his)
Graduate student
Purdue University
West Lafayette, Indiana, United States- ME
Marwa G. Elnaggar, Ph.D. (she/her/hers)
Postdoc Research Associate
Purdue University
West Lafayette, Indiana, United States 
Fanfei Meng, Ph.D. (he/him/his)
Assistant Professor
University of Massachusetts
Lowell, Massachusetts, United States
Yoon Yeo, Ph.D.
Associate Department Head, Industrial and Molecular Pharmaceutics
Purdue University
West Lafayette, Indiana, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Nucleic acids are promising drug candidates as they can address diseases with few druggable targets. Nevertheless, nucleic acids are challenging to deliver because of their unfavorable physicochemical properties and poor stability in biological fluids. Lipid nanoparticles (LNPs) have been the mainstay of nucleic acid carriers; however, systemically administered LNPs show ~80-90% accumulation in the liver [1]. Therefore, the application of systemic LNPs has mainly been limited to hepatic disorders. To develop RNA carrier for extrahepatic delivery, such as delivery to solid tumors, we developed Nanosac, a deformable and biocompatible polydopamine nanocarrier [2]. We selected CD47/SIRPa and PD-l/PD-L1 as target immune checkpoints due to their critical roles as “don't-eat-me” and “don't-find-me” signals to immune cells, respectively.
Methods: Nanosac was produced using a sacrificial template of amine-modified mesoporous silica nanoparticles (MSNa) to bind siRNA electrostatically (Fig. 1a). MSNa was coated with a polydopamine (pD) layer to protect siRNA from nucleases. Subsequently, the MSNa template was selectively removed with a dilute fluoride buffer (0.9 M) to produce hollow Nanosac. Nanosac was lyophilized with trehalose as a cryoprotectant in 1:2 w/w ratio. Cytotoxicity and protein silencing were tested in vitro in B16F10 melanoma cells. Redox-responsive siRNA release was tested in 10 mM glutathione (GSH) solution. The antitumor efficacy was evaluated in subcutaneous B16F10 melanoma and CT26 colon carcinoma mouse models in syngeneic immune-competent hosts and compared to LNPs. The treatments were administered intravenously at each siRNA dose of 0.75 mg/kg every 4 days. Nanosac biodistribution was tested in CT26 tumor-bearing mice by measuring the fluorescence intensity of Cy5-labeled siRNA in organ homogenates 6 hours after intravenous administration.
Results: In vitro characterization: Nanosac was 120 nm in diameter with a negative surface charge (Fig. 1b). Nanosac had a hollow nanocapsule structure under transmission electron microscope (TEM) (Fig. 1c). Removal of MSNa core significantly reduced the Young’s modulus of Nanosac, indicating a more deformable structure than MSNa/pD (Fig. 1d). Nanosac aggregated in electrolyte solutions but maintained its colloidal stability in presence of serum proteins or albumin, denoting the protective effect of albumin corona formation (Fig. 1e). Blank Nanosac was non-cytotoxic at therapeutic concentrations up to 1 mg/mL (Fig. 1f). Nanosac loaded with siPD-L1 or siCD47 silenced PD-L1 or CD47 immune checkpoints to a similar extent to Lipofectamine® 2000 (L2k), a cationic transfection agent (Fig. 1g). Lyophilized Nanosac maintained its colloidal stability and bioactivity, indicating the potential for cold chain-free storage and transportation (Fig. 1h). Pre-treatment of B16F10 tumor cells with siCD47-loaded Nanosac increased tumor cell phagocytosis compared to the PBS-treated control, suggesting enhanced tumor cell recognition by macrophages (Fig. 1i). Further, glutathione (GSH) promoted pD degradation and siRNA exposure from Nanosac, indicating that the elevated reductive potential inside tumor cells may mediate Nanosac biodegradation and siRNA release for target protein silencing (Fig. 1j). In vivo antitumor efficacy and biodistribution: The antitumor efficacy of Nanosac was evaluated in subcutaneous B16F10 melanoma-bearing mice representing an immune-cold tumor model. Nanosac delivering either siCD47 or siPD-L1 alone suppressed tumor growth moderately (Fig. 2a). In contrast, the mixture of two siRNA-loaded Nanosacs suppressed the tumor growth best, extending the median survival time from 16 to 26 days. Notably, with the same set of siRNAs, the Nanosac achieved greater antitumor effect than LNPs containing the ionizable lipid SM-102. An immune-hot tumor model of CT26 colon carcinoma confirmed the superior antitumor efficacy of Nanosac to LNPs (Fig. 2b). Nanosac significantly prolonged the mouse survival, with 25% of mice showing complete tumor regression. The surviving mice rejected the inoculated CT26 tumors upon rechallenging. Co-loading of the two siRNAs in the same Nanosac suppressed tumor growth to a similar extent as a mixture of separately loaded Nanosacs (Fig. 2c). Nanosac surface coating with mouse serum albumin prevented its aggregation in electrolytes in vitro (data not shown) but did not improve antitumor efficacy in vivo (Fig. 2c), suggesting that Nanosac acquired albumin corona rapidly in situ to remain stable in circulation. Moreover, Nanosac showed reduced signals of Cy5-siRNA in organs of the mononuclear phagocyte system (MPS), including liver, spleen, and lungs compared to LNPs while delivering a comparable amount of siRNA to tumors (Fig. 3).
Conclusion: Nanosac, a deformable and non-cationic carrier, was developed to fill the current gap in systemic delivery of RNA therapeutics. Nanosac silenced the target immune checkpoints in vitro and showed greater antitumor efficacy than LNPs in two mouse tumor models with reduced siRNA accumulation in the MPS organs. These results highlight the potential of Nanosac as a carrier for systemic siRNA delivery to organs beyond the liver.
References: 1. Kulkarni, J.A., et al., The current landscape of nucleic acid therapeutics. Nature Nanotechnology, 2021. 16(6): p. 630-643.
2. Kim, H., et al., Nanosac, a Noncationic and Soft Polyphenol Nanocapsule, Enables Systemic Delivery of siRNA to Solid Tumors. ACS nano, 2021. 15(3): p. 4576-4593.
Acknowledgements: We acknowledge the financial support from NIH R01 CA258737, Purdue Institute for Drug Discovery and Purdue Institute for Cancer Research for providing HG with Graduate Student Travel Award.
.jpg) Fig. 1. Preparation and in vitro characterization of Nanosac. (a) Schematic description of Nanosac preparation. (b) Size and surface charge by DLS. (c) Morphology by TEM. (d) Deformability by AFM. (e) Colloidal stability in different media. (f) Cell viability by MTT assay. (g) Checkpoint protein silencing efficiency. (h) PD-L1 silencing activity of lyophilized Nanosac. (i) Phagocytosis of B16F10 tumor cells by J774a.1 macrophages evaluated with confocal microscopy. (j) Redox-responsive siRNA release from Nanosac.
Fig. 1. Preparation and in vitro characterization of Nanosac. (a) Schematic description of Nanosac preparation. (b) Size and surface charge by DLS. (c) Morphology by TEM. (d) Deformability by AFM. (e) Colloidal stability in different media. (f) Cell viability by MTT assay. (g) Checkpoint protein silencing efficiency. (h) PD-L1 silencing activity of lyophilized Nanosac. (i) Phagocytosis of B16F10 tumor cells by J774a.1 macrophages evaluated with confocal microscopy. (j) Redox-responsive siRNA release from Nanosac.
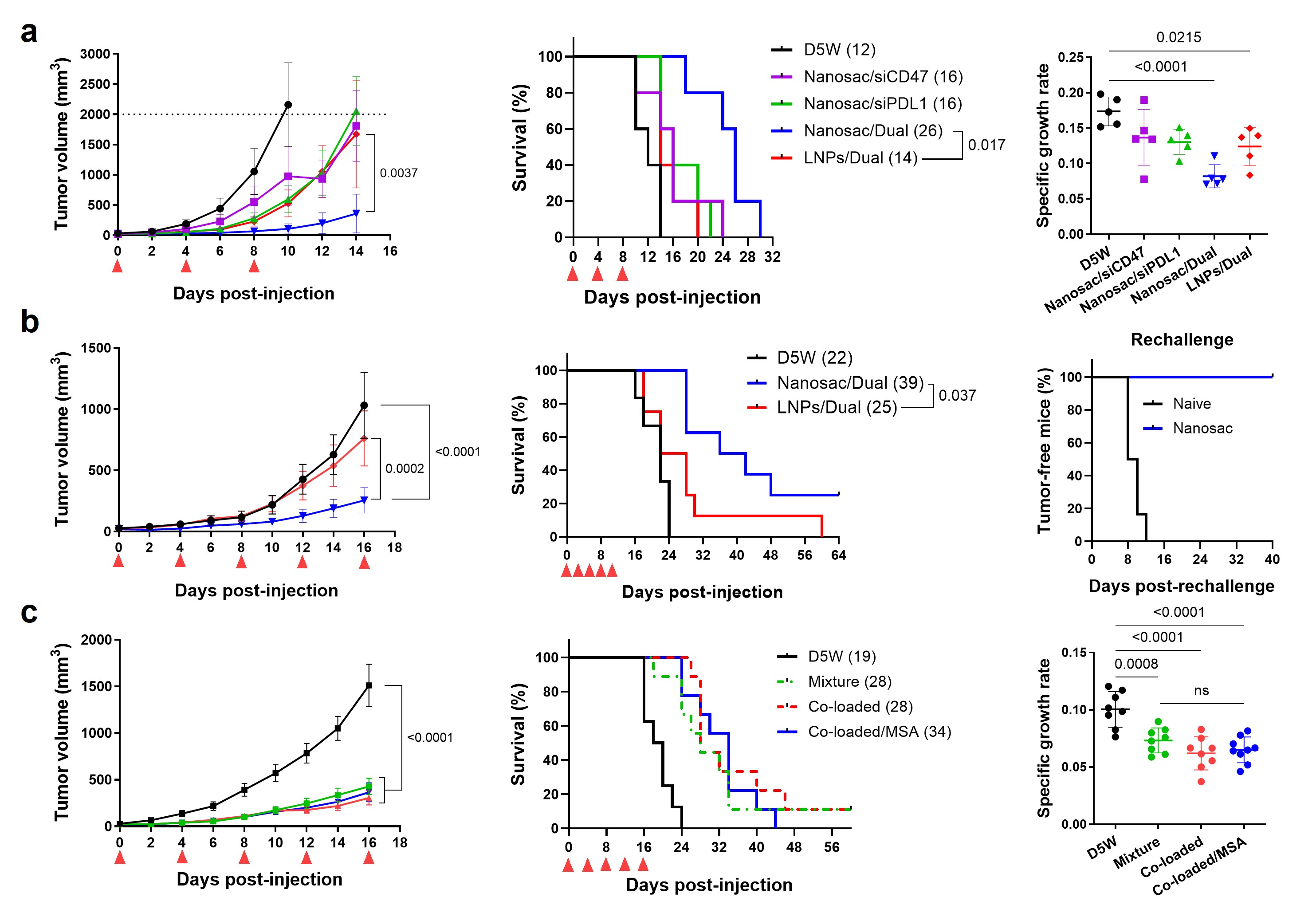 Fig. 2. In vivo antitumor efficacy of Nanosac. Antitumor efficacy in subcutaneous mouse models of (a) B16F10 melanoma or (b and c) CT26 colon carcinoma. Red arrowheads: IV dosing. Numbers in parenthesis: median survival time in days.
Fig. 2. In vivo antitumor efficacy of Nanosac. Antitumor efficacy in subcutaneous mouse models of (a) B16F10 melanoma or (b and c) CT26 colon carcinoma. Red arrowheads: IV dosing. Numbers in parenthesis: median survival time in days.
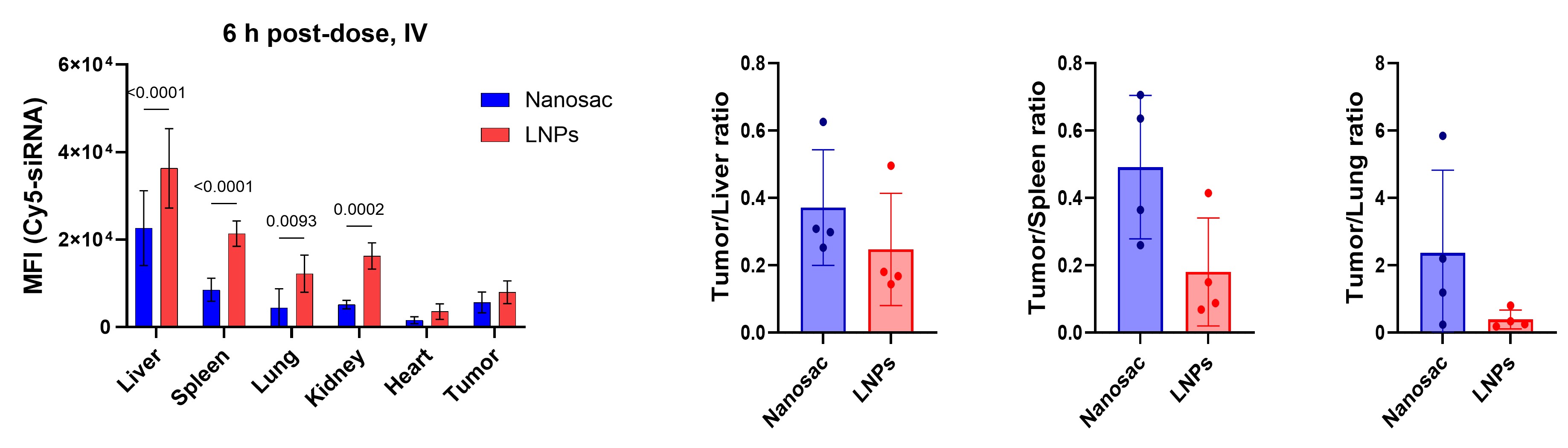 Fig. 3. Biodistribution of Cy5-siRNA loaded Nanosac or LNPs in subcutaneous CT26 tumor bearing mice 6 hours post-IV injection. Right: Mean fluorescence intensity in organ homogenates; Left: Fluorescence signal in tumors normalized to that in the MPS organs.
Fig. 3. Biodistribution of Cy5-siRNA loaded Nanosac or LNPs in subcutaneous CT26 tumor bearing mice 6 hours post-IV injection. Right: Mean fluorescence intensity in organ homogenates; Left: Fluorescence signal in tumors normalized to that in the MPS organs.
Methods: Nanosac was produced using a sacrificial template of amine-modified mesoporous silica nanoparticles (MSNa) to bind siRNA electrostatically (Fig. 1a). MSNa was coated with a polydopamine (pD) layer to protect siRNA from nucleases. Subsequently, the MSNa template was selectively removed with a dilute fluoride buffer (0.9 M) to produce hollow Nanosac. Nanosac was lyophilized with trehalose as a cryoprotectant in 1:2 w/w ratio. Cytotoxicity and protein silencing were tested in vitro in B16F10 melanoma cells. Redox-responsive siRNA release was tested in 10 mM glutathione (GSH) solution. The antitumor efficacy was evaluated in subcutaneous B16F10 melanoma and CT26 colon carcinoma mouse models in syngeneic immune-competent hosts and compared to LNPs. The treatments were administered intravenously at each siRNA dose of 0.75 mg/kg every 4 days. Nanosac biodistribution was tested in CT26 tumor-bearing mice by measuring the fluorescence intensity of Cy5-labeled siRNA in organ homogenates 6 hours after intravenous administration.
Results: In vitro characterization: Nanosac was 120 nm in diameter with a negative surface charge (Fig. 1b). Nanosac had a hollow nanocapsule structure under transmission electron microscope (TEM) (Fig. 1c). Removal of MSNa core significantly reduced the Young’s modulus of Nanosac, indicating a more deformable structure than MSNa/pD (Fig. 1d). Nanosac aggregated in electrolyte solutions but maintained its colloidal stability in presence of serum proteins or albumin, denoting the protective effect of albumin corona formation (Fig. 1e). Blank Nanosac was non-cytotoxic at therapeutic concentrations up to 1 mg/mL (Fig. 1f). Nanosac loaded with siPD-L1 or siCD47 silenced PD-L1 or CD47 immune checkpoints to a similar extent to Lipofectamine® 2000 (L2k), a cationic transfection agent (Fig. 1g). Lyophilized Nanosac maintained its colloidal stability and bioactivity, indicating the potential for cold chain-free storage and transportation (Fig. 1h). Pre-treatment of B16F10 tumor cells with siCD47-loaded Nanosac increased tumor cell phagocytosis compared to the PBS-treated control, suggesting enhanced tumor cell recognition by macrophages (Fig. 1i). Further, glutathione (GSH) promoted pD degradation and siRNA exposure from Nanosac, indicating that the elevated reductive potential inside tumor cells may mediate Nanosac biodegradation and siRNA release for target protein silencing (Fig. 1j). In vivo antitumor efficacy and biodistribution: The antitumor efficacy of Nanosac was evaluated in subcutaneous B16F10 melanoma-bearing mice representing an immune-cold tumor model. Nanosac delivering either siCD47 or siPD-L1 alone suppressed tumor growth moderately (Fig. 2a). In contrast, the mixture of two siRNA-loaded Nanosacs suppressed the tumor growth best, extending the median survival time from 16 to 26 days. Notably, with the same set of siRNAs, the Nanosac achieved greater antitumor effect than LNPs containing the ionizable lipid SM-102. An immune-hot tumor model of CT26 colon carcinoma confirmed the superior antitumor efficacy of Nanosac to LNPs (Fig. 2b). Nanosac significantly prolonged the mouse survival, with 25% of mice showing complete tumor regression. The surviving mice rejected the inoculated CT26 tumors upon rechallenging. Co-loading of the two siRNAs in the same Nanosac suppressed tumor growth to a similar extent as a mixture of separately loaded Nanosacs (Fig. 2c). Nanosac surface coating with mouse serum albumin prevented its aggregation in electrolytes in vitro (data not shown) but did not improve antitumor efficacy in vivo (Fig. 2c), suggesting that Nanosac acquired albumin corona rapidly in situ to remain stable in circulation. Moreover, Nanosac showed reduced signals of Cy5-siRNA in organs of the mononuclear phagocyte system (MPS), including liver, spleen, and lungs compared to LNPs while delivering a comparable amount of siRNA to tumors (Fig. 3).
Conclusion: Nanosac, a deformable and non-cationic carrier, was developed to fill the current gap in systemic delivery of RNA therapeutics. Nanosac silenced the target immune checkpoints in vitro and showed greater antitumor efficacy than LNPs in two mouse tumor models with reduced siRNA accumulation in the MPS organs. These results highlight the potential of Nanosac as a carrier for systemic siRNA delivery to organs beyond the liver.
References: 1. Kulkarni, J.A., et al., The current landscape of nucleic acid therapeutics. Nature Nanotechnology, 2021. 16(6): p. 630-643.
2. Kim, H., et al., Nanosac, a Noncationic and Soft Polyphenol Nanocapsule, Enables Systemic Delivery of siRNA to Solid Tumors. ACS nano, 2021. 15(3): p. 4576-4593.
Acknowledgements: We acknowledge the financial support from NIH R01 CA258737, Purdue Institute for Drug Discovery and Purdue Institute for Cancer Research for providing HG with Graduate Student Travel Award.
.jpg) Fig. 1. Preparation and in vitro characterization of Nanosac. (a) Schematic description of Nanosac preparation. (b) Size and surface charge by DLS. (c) Morphology by TEM. (d) Deformability by AFM. (e) Colloidal stability in different media. (f) Cell viability by MTT assay. (g) Checkpoint protein silencing efficiency. (h) PD-L1 silencing activity of lyophilized Nanosac. (i) Phagocytosis of B16F10 tumor cells by J774a.1 macrophages evaluated with confocal microscopy. (j) Redox-responsive siRNA release from Nanosac.
Fig. 1. Preparation and in vitro characterization of Nanosac. (a) Schematic description of Nanosac preparation. (b) Size and surface charge by DLS. (c) Morphology by TEM. (d) Deformability by AFM. (e) Colloidal stability in different media. (f) Cell viability by MTT assay. (g) Checkpoint protein silencing efficiency. (h) PD-L1 silencing activity of lyophilized Nanosac. (i) Phagocytosis of B16F10 tumor cells by J774a.1 macrophages evaluated with confocal microscopy. (j) Redox-responsive siRNA release from Nanosac. Fig. 2. In vivo antitumor efficacy of Nanosac. Antitumor efficacy in subcutaneous mouse models of (a) B16F10 melanoma or (b and c) CT26 colon carcinoma. Red arrowheads: IV dosing. Numbers in parenthesis: median survival time in days.
Fig. 2. In vivo antitumor efficacy of Nanosac. Antitumor efficacy in subcutaneous mouse models of (a) B16F10 melanoma or (b and c) CT26 colon carcinoma. Red arrowheads: IV dosing. Numbers in parenthesis: median survival time in days. Fig. 3. Biodistribution of Cy5-siRNA loaded Nanosac or LNPs in subcutaneous CT26 tumor bearing mice 6 hours post-IV injection. Right: Mean fluorescence intensity in organ homogenates; Left: Fluorescence signal in tumors normalized to that in the MPS organs.
Fig. 3. Biodistribution of Cy5-siRNA loaded Nanosac or LNPs in subcutaneous CT26 tumor bearing mice 6 hours post-IV injection. Right: Mean fluorescence intensity in organ homogenates; Left: Fluorescence signal in tumors normalized to that in the MPS organs.