Formulation and Delivery - Chemical
(T1230-10-59) Elucidation of Drug Release Mechanisms of Quetiapine Fumarate Extended-Release Tablets Via Real Time Surface Dissolution Imaging
Tuesday, October 22, 2024
12:30 PM - 1:30 PM MT

Weizhou Yue, PhD
Postdoctoral Fellow
Northeastern University
Boston, Massachusetts, United States
Weizhou Yue, PhD
Postdoctoral Fellow
Northeastern University
Boston, Massachusetts, United States- HC
Hyeongeun (Tony) Cho, n/a
PharmD Candidate
Northeastern University
Boston, Massachusetts, United States - XP
Xavier Pepin, n/a
Vice President
Simulations Plus, Inc.
Lancaster, California, United States 
Jie Shen, n/a (she/her/hers)
Associate Professor
Northeastern University
Boston, Massachusetts, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Oral extended-release (ER) drug products, featuring controlled drug release characteristics (e.g., rate, duration), have been extensively utilized to attain desired therapeutic outcomes, diminish adverse effects, and/or enhance patient adherence compared to conventional oral solid dosage forms. Compared to the bio-strength, drugs and excipients in different strengths of oral ER tablets can be either proportional or non-proportional in quantity. Currently, appropriate factors for scaling oral ER tablet formulations for additional strengths have yet to be identified. To this end, it is essential to understand drug release mechanism(s) and critical materials attributes and manufacturing process parameters for such products. The present research aims to elucidate drug release mechanism(s) of the selected ER tablet across multiple strengths through comparative characterization and dissolution technologies.
Methods: Quetiapine fumarate (QF) ER tablets with multiple strengths (i.e., 50, 150, 200, 300, and 400 mg) were chosen as model drug products. Quality attributes (e.g., tablet weight, dimensions, hardness, inner structure, drug and excipient distribution) of the QF ER tablets were characterized. In vitro dissolution testing of the QF ER tablets were conducted at 37°C in biphasic media using USP Apparatus 1 (Basket, n=3). Dissolution samples were withdrawn at pre-determined time points and analyzed using a validated high performance liquid chromatography (HPLC) method. Dissolution parameters such as medium and basket mesh size were investigated. For the comparison of dissolution profiles, the similarity factor (f2) was calculated. Moreover, real-time tablet matrix changes and QF release were monitored in biphasic media at 37°C via a surface dissolution imaging system (SDi2).
Results: Different strengths of the QF ER tablet products had different weight, dimensions, and hardness. The QF ER tablets across different strengths showed a similar matrix structure (e.g., porosity) and drug/excipient distribution based on X-ray microscopy (XRM) and Raman imaging studies, respectively. More complete release was obtained when biphasic media and 20-mesh basket were used compared to that obtained using the 40-mesh basket. The in vitro dissolution studies indicated that QF ER tablet products with different strengths had different dissolution profiles (Figure 1, f2 < 50). Moreover, obvious matrix swelling of the reference bio-strength (200 mg) was visualized during the biphasic dissolution study (Figure 2). Changes in tablet the height (ΔH) and width (ΔW) were 1.25 and 2.14 mm at around 2 hours and 4.38 and 10.40 mm at around 14 hours, respectively.
Conclusion: We have developed comparatively characterization and dissolution methodologies for QF ER tablet products. A typical matrix-swelling driven QF release was observed. Additional research is planned to evaluate key formulation and process variables for QF ER tablets across multiple strengths, and to better understand the influence of the dynamic gastrointestinal environment under fasted and fed conditions on the in vitro dissolution performance of QF ER tablets.
Acknowledgements: This project is supported by the FDA of the U.S. Department of Health and Human Services (HHS) under grant award 1U01FD007959-01. The views are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government. We would like to thank Liyuan Gong and Dr. Matthew Cabral from the University of Rhode Island for their assistance with the XRM and Raman microscopy studies.
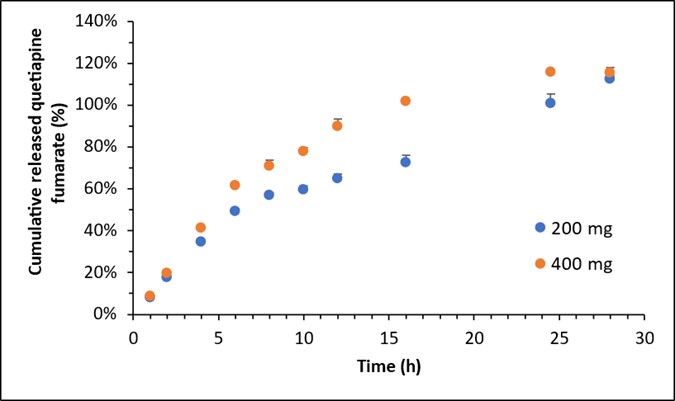 Figure 1. The in vitro dissolution profiles of QF ER tablet products with 200 and 400 mg strengths obtained using USP apparatus 1 at 37°C in biphasic media (0.05M citric acid and 0.09 N NaOH, pH 4.8 for first 5 hours followed by the addition of 100 mL of 0.05M dibasic sodium phosphate dodecahydrate and 0.46 N NaOH to reach a final medium pH of 6.7) (n=3, mean±SD).
Figure 1. The in vitro dissolution profiles of QF ER tablet products with 200 and 400 mg strengths obtained using USP apparatus 1 at 37°C in biphasic media (0.05M citric acid and 0.09 N NaOH, pH 4.8 for first 5 hours followed by the addition of 100 mL of 0.05M dibasic sodium phosphate dodecahydrate and 0.46 N NaOH to reach a final medium pH of 6.7) (n=3, mean±SD).
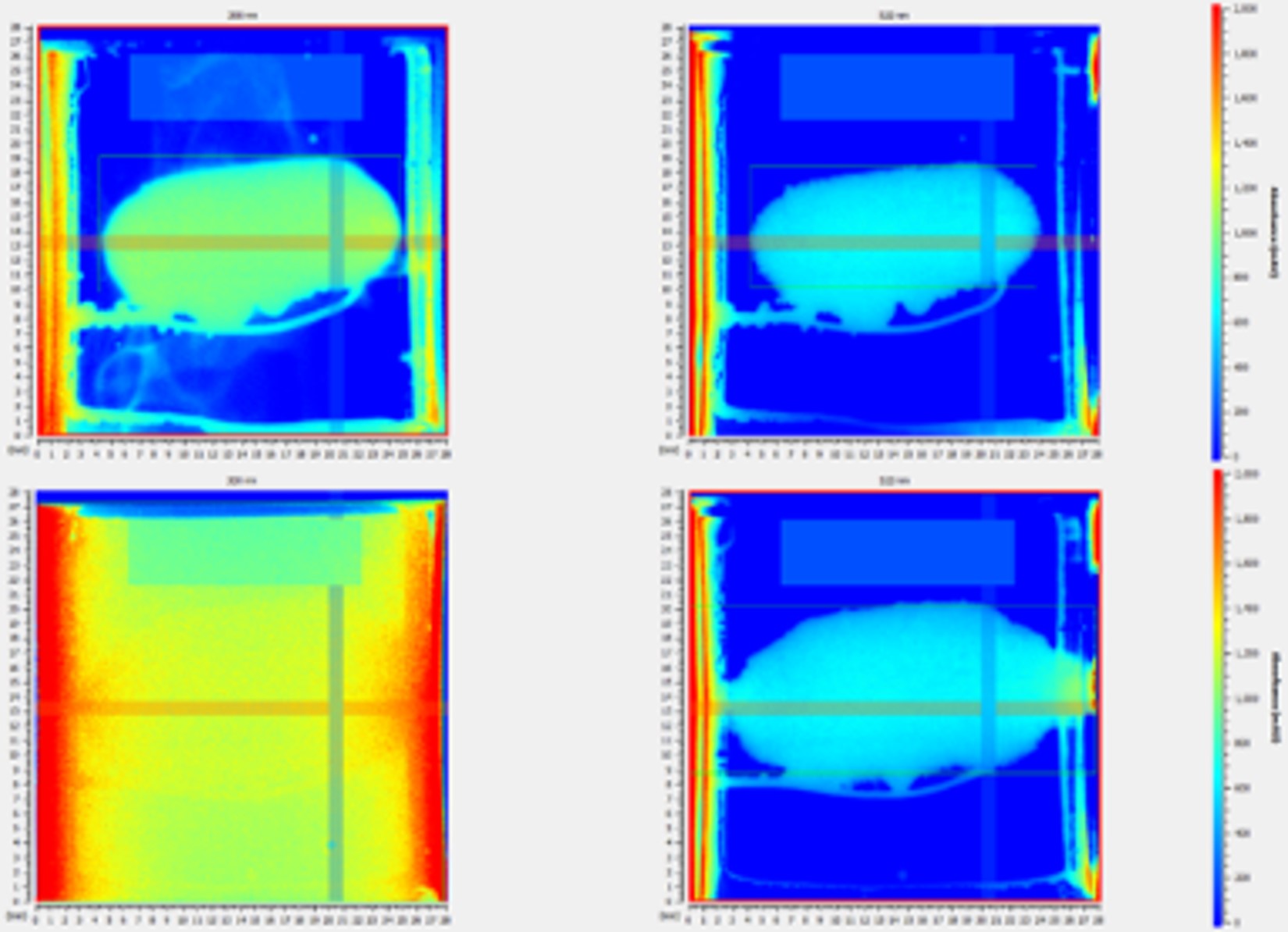 Figure 2. Representative surface dissolution images (left: UV channel, right: visible light channel) at around 2 hours (upper panel) and 14 hours (lower panel) of QF ER tablet product (200 mg strength) obtained using SDi2 at 37°C in biphasic media (Media A: 8.0 g/L sodium chloride, 1.7 g/L potassium chloride, 0.16 g/L calcium chloride dihydrate, pH 3.0 for first 2 hours; Media B: 7 g/L sodium chloride, 35 g/L potassium chloride, 0.1 g/L calcium chloride dihydrate, pH 6.7 after 2 hours).
Figure 2. Representative surface dissolution images (left: UV channel, right: visible light channel) at around 2 hours (upper panel) and 14 hours (lower panel) of QF ER tablet product (200 mg strength) obtained using SDi2 at 37°C in biphasic media (Media A: 8.0 g/L sodium chloride, 1.7 g/L potassium chloride, 0.16 g/L calcium chloride dihydrate, pH 3.0 for first 2 hours; Media B: 7 g/L sodium chloride, 35 g/L potassium chloride, 0.1 g/L calcium chloride dihydrate, pH 6.7 after 2 hours).
Methods: Quetiapine fumarate (QF) ER tablets with multiple strengths (i.e., 50, 150, 200, 300, and 400 mg) were chosen as model drug products. Quality attributes (e.g., tablet weight, dimensions, hardness, inner structure, drug and excipient distribution) of the QF ER tablets were characterized. In vitro dissolution testing of the QF ER tablets were conducted at 37°C in biphasic media using USP Apparatus 1 (Basket, n=3). Dissolution samples were withdrawn at pre-determined time points and analyzed using a validated high performance liquid chromatography (HPLC) method. Dissolution parameters such as medium and basket mesh size were investigated. For the comparison of dissolution profiles, the similarity factor (f2) was calculated. Moreover, real-time tablet matrix changes and QF release were monitored in biphasic media at 37°C via a surface dissolution imaging system (SDi2).
Results: Different strengths of the QF ER tablet products had different weight, dimensions, and hardness. The QF ER tablets across different strengths showed a similar matrix structure (e.g., porosity) and drug/excipient distribution based on X-ray microscopy (XRM) and Raman imaging studies, respectively. More complete release was obtained when biphasic media and 20-mesh basket were used compared to that obtained using the 40-mesh basket. The in vitro dissolution studies indicated that QF ER tablet products with different strengths had different dissolution profiles (Figure 1, f2 < 50). Moreover, obvious matrix swelling of the reference bio-strength (200 mg) was visualized during the biphasic dissolution study (Figure 2). Changes in tablet the height (ΔH) and width (ΔW) were 1.25 and 2.14 mm at around 2 hours and 4.38 and 10.40 mm at around 14 hours, respectively.
Conclusion: We have developed comparatively characterization and dissolution methodologies for QF ER tablet products. A typical matrix-swelling driven QF release was observed. Additional research is planned to evaluate key formulation and process variables for QF ER tablets across multiple strengths, and to better understand the influence of the dynamic gastrointestinal environment under fasted and fed conditions on the in vitro dissolution performance of QF ER tablets.
Acknowledgements: This project is supported by the FDA of the U.S. Department of Health and Human Services (HHS) under grant award 1U01FD007959-01. The views are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government. We would like to thank Liyuan Gong and Dr. Matthew Cabral from the University of Rhode Island for their assistance with the XRM and Raman microscopy studies.
 Figure 1. The in vitro dissolution profiles of QF ER tablet products with 200 and 400 mg strengths obtained using USP apparatus 1 at 37°C in biphasic media (0.05M citric acid and 0.09 N NaOH, pH 4.8 for first 5 hours followed by the addition of 100 mL of 0.05M dibasic sodium phosphate dodecahydrate and 0.46 N NaOH to reach a final medium pH of 6.7) (n=3, mean±SD).
Figure 1. The in vitro dissolution profiles of QF ER tablet products with 200 and 400 mg strengths obtained using USP apparatus 1 at 37°C in biphasic media (0.05M citric acid and 0.09 N NaOH, pH 4.8 for first 5 hours followed by the addition of 100 mL of 0.05M dibasic sodium phosphate dodecahydrate and 0.46 N NaOH to reach a final medium pH of 6.7) (n=3, mean±SD). Figure 2. Representative surface dissolution images (left: UV channel, right: visible light channel) at around 2 hours (upper panel) and 14 hours (lower panel) of QF ER tablet product (200 mg strength) obtained using SDi2 at 37°C in biphasic media (Media A: 8.0 g/L sodium chloride, 1.7 g/L potassium chloride, 0.16 g/L calcium chloride dihydrate, pH 3.0 for first 2 hours; Media B: 7 g/L sodium chloride, 35 g/L potassium chloride, 0.1 g/L calcium chloride dihydrate, pH 6.7 after 2 hours).
Figure 2. Representative surface dissolution images (left: UV channel, right: visible light channel) at around 2 hours (upper panel) and 14 hours (lower panel) of QF ER tablet product (200 mg strength) obtained using SDi2 at 37°C in biphasic media (Media A: 8.0 g/L sodium chloride, 1.7 g/L potassium chloride, 0.16 g/L calcium chloride dihydrate, pH 3.0 for first 2 hours; Media B: 7 g/L sodium chloride, 35 g/L potassium chloride, 0.1 g/L calcium chloride dihydrate, pH 6.7 after 2 hours).