Formulation and Delivery - Chemical
(T1530-09-49) Untangling the Interplay of Drug Solubility, Microstructure, and Polymer Behavior on the Performance of Commercial Controlled Release Microsphere Products

Andrew G. Clark, PhD (he/him/his)
Director of Pharmaceutical Science
digiM Solution
Woburn, Massachusetts, United States
Andrew G. Clark, PhD (he/him/his)
Director of Pharmaceutical Science
digiM Solution
Woburn, Massachusetts, United States- RW
Ruifeng Wang, BS
Research Assistant
University of Connecticut
Storrs, Connecticut, United States - JG
Jonah Gautreau, MS
Application Scientist
DigiM Solution LLC
Woburn, Massachusetts, United States - YW
Yan Wang, Ph.D. (she/her/hers)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States 
Bin Qin, Ph.D.
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States
Diane Burgess, Ph.D.
Board of Trustees Distinguished Professor of Pharmaceutics Pfizer Distinguished Chair of Pharmaceuti
University of Connecticut
Storrs, Connecticut, United States
Shawn Zhang, Ph.D. (he/him/his)
Co-founder, Managing Director
DigiM Solution LLC
Woburn, Massachusetts, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Risperdal Consta® (risperidone, low aqueous solubility) and Arestin® (minocycline hydrochloride, high aqueous solubility) were selected as the model RLDs. In addition, two in-house Q1Q2 formulations of each RLD were prepared and investigated. Table 1 shows the different characteristics of all the formulations. The microstructures of the different formulations were quantified using focused ion beam scanning electron microscopy (FIB-SEM). Different structural CQAs including phase fractions of PLGA, porosity and API, size distributions of the phases, and spatial distributions were determined from imaging. In vitro release testing was performed for each microsphere product for assessment of downstream performance.
Results: Figure 1 shows the in vitro release profiles for the risperidone loaded microspheres (Figure 1a) and the minocycline hydrochloride loaded microspheres (Figure 1b). The risperidone microspheres show multiphasic release lasting around 30 - 40 days with obvious lag phases, while the minocycline hydrochloride loaded microspheres show high initial burst release, and overall release lasting ~14 days. Figure 2 shows representative FIB-SEM cross sections of each microsphere type. Both microsphere types show phase separated API particles within the PLGA as well as extensive porosity throughout. Comparison between the RLDs and their Q1Q2 in-house test formulations show significant differences in their internal porosities and pore spatial distributions for both products indicating major structural differences. For the minocycline hydrochloride-loaded microspheres, the variation in porosity appears to promote significant variations in release performance with the more porous microspheres releasing faster. In contrast, for the risperidone-loaded systems, release from the less porous RM-2 is significantly faster than the more porous RM-1, which may be due to the different polymer properties (e.g., molecular weight, blockiness) as well as microstructural properties of the microspheres [4]. This may indicate a dynamic relationship between drug solubility and internal microstructure for these microsphere systems.
Conclusion: Utilizing the next generation of quantitative imaging including FIB-SEM, the microstructure of two different microsphere systems was characterized in an effort to understand the interplay between drugs of different physicochemical properties (e.g., aqueous solubility) and microsphere microstructure. In the case of minocycline hydrochloride, the microsphere internal structure appears to have a strong impact on drug release, with a more porous structure resulting in faster release. For risperidone system, the microstructure as well as the PLGA properties impact the release performance for this class of microsphere. Overall, this research offers a deep insight into assessing sameness in microsphere systems and may facilitate the development of generic microspheres products using a more data driven quality-by-design approach.
References: [1] A.G. Clark, R. Wang, Y. Qin, Y. Wang, A. Zhu, J. Lomeo, Q. Bao, D.J. Burgess, J. Chen, B. Qin, Assessing microstructural critical quality attributes in PLGA microspheres by FIB-SEM analytics, J. Control. Release. 349 (2022) 580–591.
[2] A.G. Clark, R. Wang, J. Lomeo, Y. Wang, A. Zhu, M. Shen, Q. Bao, D.J. Burgess, B. Qin, S. Zhang, Investigating structural attributes of drug encapsulated microspheres with quantitative X-ray imaging, J. Control. Release. 358 (2023) 626–635.
[3] R. Wang, Q. Bao, A.G. Clark, Y. Wang, S. Zhang, D.J. Burgess, Characterization and in vitro release of minocycline hydrochloride microspheres prepared via coacervation, Int. J. Pharm. 628 (2022) 122292. https://doi.org/https://doi.org/10.1016/j.ijpharm.2022.122292.
[4] B. Wan, Q. Bao, Y. Zou, Y. Wang, D.J. Burgess, Effect of polymer source variation on the properties and performance of risperidone microspheres, Int. J. Pharm. 610 (2021) 121265.
Acknowledgements: This work was partially supported by the Broad Agency Announcement (BAA) Contract # 75F40122C00163 from the U.S. Food and Drug Administration (FDA). The content is solely the responsibility of the authors and does not necessarily represent the official views or policies of the U.S. FDA.
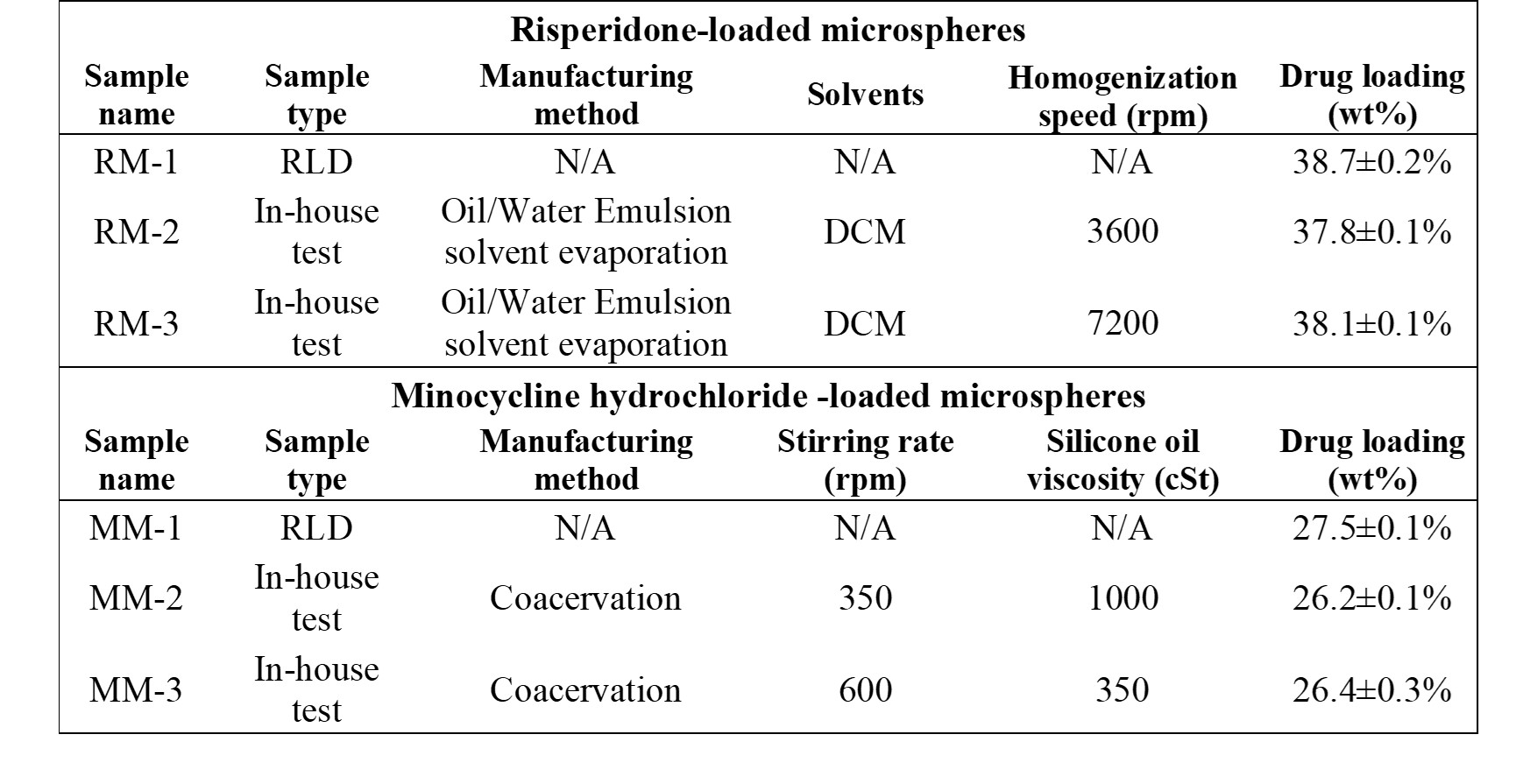 Table 1. Manufacturing characteristics for risperidone and minocycline hydrochloride loaded microspheres
Table 1. Manufacturing characteristics for risperidone and minocycline hydrochloride loaded microspheres 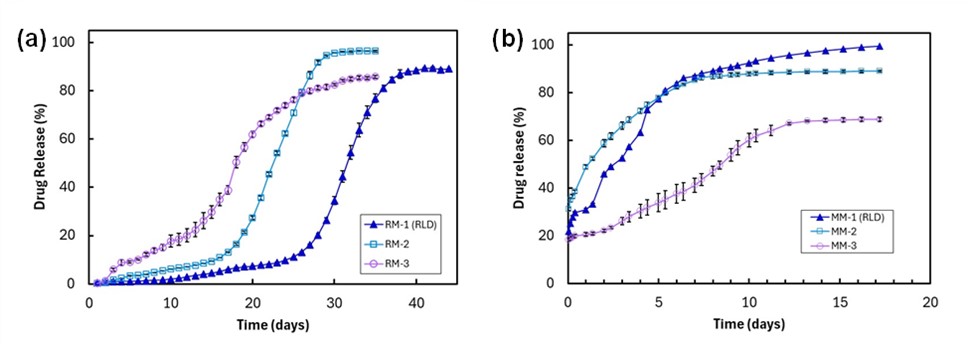 Figure 1. In vitro release profiles for risperidone loaded microspheres (a), and minocycline hydrochloride loaded microspheres (b).
Figure 1. In vitro release profiles for risperidone loaded microspheres (a), and minocycline hydrochloride loaded microspheres (b).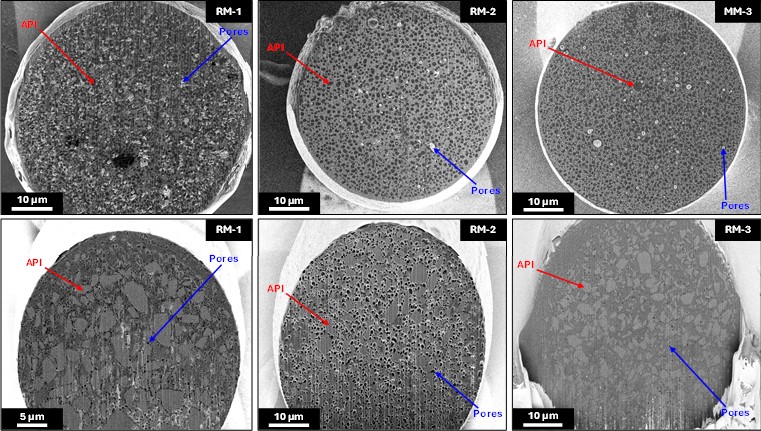 Figure 2. FIB-SEM images of the risperidone loaded microspheres (top row) and minocycline hydrochloride loaded microspheres (bottom row). API particles and pores are indicated for each microsphere. The greyscale contrast swaps between the two different microsphere species due to variations in the optimal imaging parameters as well as the different electron structures of the API molecules.
Figure 2. FIB-SEM images of the risperidone loaded microspheres (top row) and minocycline hydrochloride loaded microspheres (bottom row). API particles and pores are indicated for each microsphere. The greyscale contrast swaps between the two different microsphere species due to variations in the optimal imaging parameters as well as the different electron structures of the API molecules.