Manufacturing and Analytical Characterization - Chemical
(T1530-11-62) The Necessity of Using LC-MS/MS Technology to Investigate the Presence of N-Nitrosamines Impurities in Pharmaceutical Drug Products
Tuesday, October 22, 2024
3:30 PM - 4:30 PM MT
- FD
Frank De Smedt, Ph.D.
Director of Scientific Improvements
Nelson Labs
Leuven, Vlaams-Brabant, Belgium - FD
Frank De Smedt, Ph.D.
Director of Scientific Improvements
Nelson Labs
Leuven, Vlaams-Brabant, Belgium - AR
Ank Reumer, Sr., Ph.D.
Sr Study Director
Nelson Labs
leuven, Vlaams-Brabant, Belgium - MC
Matt Cushing, MS
VP of Quality and Science
Nelson Labs
salt lake city, Utah, United States - AN
Aryo Nikopour, MS
VP Global Market Leader, Pharmaceutical
Nelson Labs
salt lake city, Utah, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Since 2018 there has been an increased attention from regulatory authorities towards the possible presence of nitrosamine impurities in human medicinal products containing chemically synthesized active pharmaceutical ingredients and biologically active substances. The focus on N-Nitrosamines is triggered by the fact that these chemical compounds are classified as probable human carcinogens and exhibit a greater correlation between mutagenicity and carcinogenicity compared to non-nitrosamine compounds. Nitrosamines are formed due to a specific chemical reaction whereby both nitrites, known to be oxidizing by nature, and amines (functional group) are present in specific conditions. Currently identified sources of nitrosamine impurities include the use or presence of nitrites in production processes, contaminated raw materials, cross contamination, degradation reactions, the use of certain packaging materials: Figure 1. Important to note that the potential source isn’t only the chemical formation during the DP manufacturing process. The European Medicines Agency (EMA) has defined a process with in a first step a thorough risk assessment and if needed, in step 2 confirmatory testing. The latter is a challenging analytical requirement as not only the appropriate mass spectrometry based equipment should be used, but also the detection limits (low) and the matrix (challenging) contribute to this analytical complexity. For the US market the FDA has mainly followed the EMA focus and attention on the N-Nitrosamines in finished DP with some small differences in its approach e.g. on the limits to be applied for the analytical testing. The identification and subsequent quantification (when needed) is an analytical challenge requiring state-of-the-art equipment, e.g. a Liquid Chromatography Triple Quad MS system, exhibiting enough analytical sensitivity for the most difficult N-Nitrosamines whereby the latter is often a combination of the intrinsic (low) sensitivity, the low thresholds (application and dose depending) and potential matrix interferences. An experimental case is described to illustrate the challenges in N-Nitrosamine testing in drug products as well as the need of LC-MS/MS equipment and appropriate, optimized methodologies. The importance of the establishment of the AET concentration is also demonstrated.
Methods: Standard solutions of nitrosamines are purchased commercially with a CoA. Solvents used in the chromatographic analysis and in the preparation of standards or spike solutions are of high quality grades to reduce the background presence of the target species. The equipment used in these case studies is an Agilent LC-MS/MS with Masshunter Acq software 10.1.
Results: The first challenge in each study on N-Nitrosamines concerns the establishment of the analytical evaluation threshold (AET) is deduced from the Acceptable Intake (AI) Limit specific for each N-Nitrosamine (Table 1) and the maximum daily dose (MDD) which is application dependent. The AET concentration calculated guides the development (and potential validation) of the method for the detection and confirmation of a specific N-Nitrosamine in the sample, e.g. the challenge in specificity/selectivity. Table 1 provides some AI limits for common N-Nitrosamines and a calculation of the AET concentration in various applications, e.g. Large Volume Parenterals (LVP), IV bags or syringes used for the administration of vaccines. It’s clear the key parameter, next to the AI limit, is the established daily dose leading to target analytical concentrations from as high as 26.5 µg/L to ultra trace concentrations of 0.5 ng/L. N-Nitrosodiproplyamine and N-Nitrosopiperidine (Fig 2) were at first the subject of a method development/feasibility study leading to the definition of the appropriate column to obtain sufficient separation, the ionization mode (APCI or ESI, positive or negative) and MRM transitions.Peak separation was obtained and subsequently a matrix spike was compared to the standard in solvent for the same species to assess specificity requirements. From the examples in Figure 2 it’s obvious that the selection of the appropriate column to separate the multiple N-Nitrosamines peaks is important, but more crucial is selecting the right MRM transitions to guarantee specificity and ultimately being able to distinguish between the actual presence of a target N-Nitrosamine and a false positive. Other challenges are a.o. the search for a suitable sample preparation procedure, establishing a realistic concentration factor, selection of a suited extraction solvent, the internal standard(s), evaluation on background presence of the target species. Potential sources are e.g. gloves, solvents, filters, polymeric materials used for the container closure systems.
Conclusion: Risk evaluation of all data available (e.g. E&L data) is the first step, a focus on drug products with high daily doses should be the next as these exhibit the lowest analytical evaluation threshold concentration. In view of the analytical challenges special attention should be given to the appropriate instrument (LC-MS/MS) and analytical column to obtain sufficient sensitivity and peak separation. Specificity is obvious crucial in this analysis to positively identify any present N-Nitrosamine and more challenges could be present in case of low AET levels, complex drug product matrix and respective mass spectral interferences with the selection of the appropriate MRM settings being key in the latter.
References: EMA updated Appendix 1 on “Acceptable Intakes (Ais) for N-nitrosamines” on 11th May 2024, EMA/15440/2024/rev.4 (NcWP; non-clinical Working Group)
FDA‘s Control of Nitrosamine Impurities in Human Drugs (Guidance for industry)
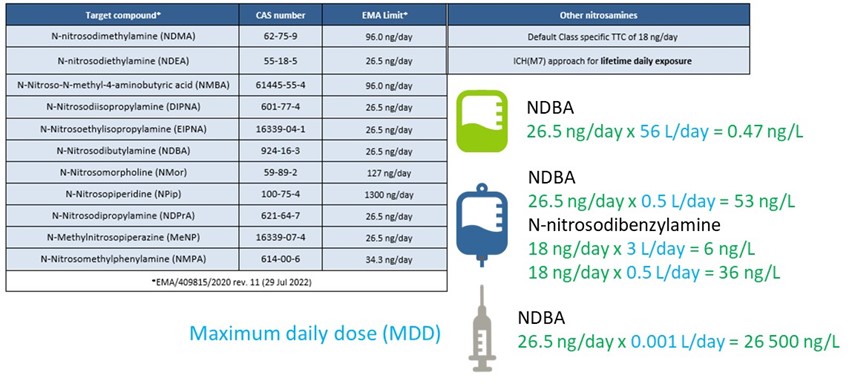 Table 1: Selected N-Nitrosamines and their Acceptable Intake limit (EMA limit) with examples of calculating the AET as a function of the application.
Table 1: Selected N-Nitrosamines and their Acceptable Intake limit (EMA limit) with examples of calculating the AET as a function of the application.
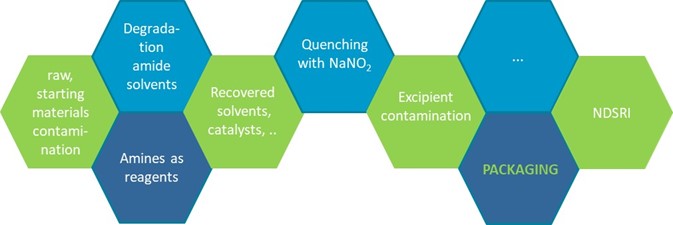 Figure 1: Visualization of the potential sources of N-Nitrosamines in pharmaceutical drug products.
Figure 1: Visualization of the potential sources of N-Nitrosamines in pharmaceutical drug products.
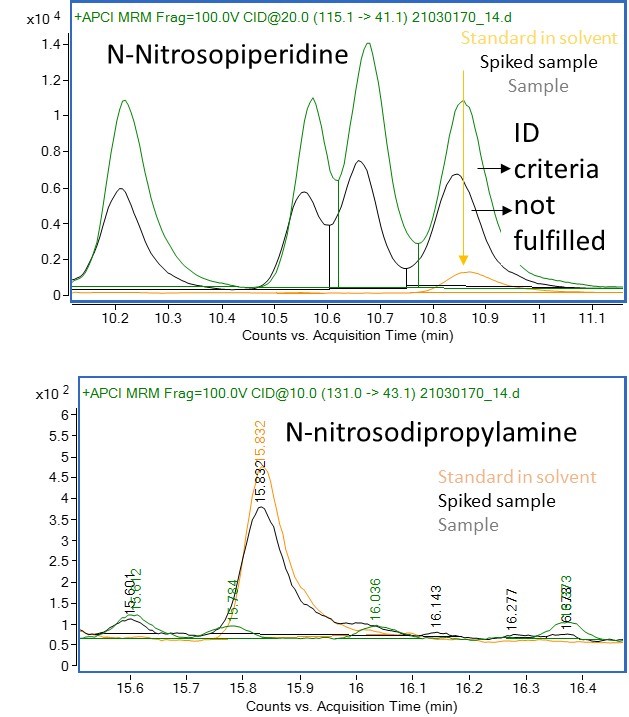 Figure 2: LC-MS/MS analysis of a drug product (after appropriate sample prep) with APCI + mode (sample, spiked sample and standard in solvent) for N-Nitrosopiperidine (upper; MRM from 115.1 to 41.1) and N-Nitrosopropylamine (lower; MRM from 131.0 to 43.1).
Figure 2: LC-MS/MS analysis of a drug product (after appropriate sample prep) with APCI + mode (sample, spiked sample and standard in solvent) for N-Nitrosopiperidine (upper; MRM from 115.1 to 41.1) and N-Nitrosopropylamine (lower; MRM from 131.0 to 43.1).
Methods: Standard solutions of nitrosamines are purchased commercially with a CoA. Solvents used in the chromatographic analysis and in the preparation of standards or spike solutions are of high quality grades to reduce the background presence of the target species. The equipment used in these case studies is an Agilent LC-MS/MS with Masshunter Acq software 10.1.
Results: The first challenge in each study on N-Nitrosamines concerns the establishment of the analytical evaluation threshold (AET) is deduced from the Acceptable Intake (AI) Limit specific for each N-Nitrosamine (Table 1) and the maximum daily dose (MDD) which is application dependent. The AET concentration calculated guides the development (and potential validation) of the method for the detection and confirmation of a specific N-Nitrosamine in the sample, e.g. the challenge in specificity/selectivity. Table 1 provides some AI limits for common N-Nitrosamines and a calculation of the AET concentration in various applications, e.g. Large Volume Parenterals (LVP), IV bags or syringes used for the administration of vaccines. It’s clear the key parameter, next to the AI limit, is the established daily dose leading to target analytical concentrations from as high as 26.5 µg/L to ultra trace concentrations of 0.5 ng/L. N-Nitrosodiproplyamine and N-Nitrosopiperidine (Fig 2) were at first the subject of a method development/feasibility study leading to the definition of the appropriate column to obtain sufficient separation, the ionization mode (APCI or ESI, positive or negative) and MRM transitions.Peak separation was obtained and subsequently a matrix spike was compared to the standard in solvent for the same species to assess specificity requirements. From the examples in Figure 2 it’s obvious that the selection of the appropriate column to separate the multiple N-Nitrosamines peaks is important, but more crucial is selecting the right MRM transitions to guarantee specificity and ultimately being able to distinguish between the actual presence of a target N-Nitrosamine and a false positive. Other challenges are a.o. the search for a suitable sample preparation procedure, establishing a realistic concentration factor, selection of a suited extraction solvent, the internal standard(s), evaluation on background presence of the target species. Potential sources are e.g. gloves, solvents, filters, polymeric materials used for the container closure systems.
Conclusion: Risk evaluation of all data available (e.g. E&L data) is the first step, a focus on drug products with high daily doses should be the next as these exhibit the lowest analytical evaluation threshold concentration. In view of the analytical challenges special attention should be given to the appropriate instrument (LC-MS/MS) and analytical column to obtain sufficient sensitivity and peak separation. Specificity is obvious crucial in this analysis to positively identify any present N-Nitrosamine and more challenges could be present in case of low AET levels, complex drug product matrix and respective mass spectral interferences with the selection of the appropriate MRM settings being key in the latter.
References: EMA updated Appendix 1 on “Acceptable Intakes (Ais) for N-nitrosamines” on 11th May 2024, EMA/15440/2024/rev.4 (NcWP; non-clinical Working Group)
FDA‘s Control of Nitrosamine Impurities in Human Drugs (Guidance for industry)
 Table 1: Selected N-Nitrosamines and their Acceptable Intake limit (EMA limit) with examples of calculating the AET as a function of the application.
Table 1: Selected N-Nitrosamines and their Acceptable Intake limit (EMA limit) with examples of calculating the AET as a function of the application. Figure 1: Visualization of the potential sources of N-Nitrosamines in pharmaceutical drug products.
Figure 1: Visualization of the potential sources of N-Nitrosamines in pharmaceutical drug products. Figure 2: LC-MS/MS analysis of a drug product (after appropriate sample prep) with APCI + mode (sample, spiked sample and standard in solvent) for N-Nitrosopiperidine (upper; MRM from 115.1 to 41.1) and N-Nitrosopropylamine (lower; MRM from 131.0 to 43.1).
Figure 2: LC-MS/MS analysis of a drug product (after appropriate sample prep) with APCI + mode (sample, spiked sample and standard in solvent) for N-Nitrosopiperidine (upper; MRM from 115.1 to 41.1) and N-Nitrosopropylamine (lower; MRM from 131.0 to 43.1).