Formulation and Delivery - Chemical
(M1030-10-56) Development and Validation of Biorelevant Multimodal In Vitro Characterization Techniques for Assessing the Adhesiveness Performance of Transdermal Patches
Monday, October 21, 2024
10:30 AM - 11:30 AM MT
- SK
Sumedha Kapre, MS
Graduate Student
Texas A&M University
KINGSVILLE, Texas, United States - SK
Sumedha Kapre, MS
Graduate Student
Texas A&M University
KINGSVILLE, Texas, United States - SP
Sushesh Srivatsa Palakurthi, MS
Graduate Student
Texas A&M University
Kingsville, Texas, United States - MV
Mrudul Velhal, MS
Graduate Student
Texas A&M University
College Station, Texas, United States - HL
Hong Liang, Ph.D.
Professor
Texas A&M University
College Station, Texas, United States - WZ
Wei Zheng, Ph.D.
Assistant Professor
Texas A&M University
Corpus Christi, Texas, United States - SP
Srinath Palakurthi, Ph.D. (he/him/his)
Professor
Texas A&M University
Kingsville, Texas, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Adhesion is a pivotal determinant of the quality, efficacy, and safety of transdermal patches. Although various in vitro techniques (e.g., peel adhesion, tack, and shear strength assessments) are routinely employed, human skin testing remains the gold standard for evaluating these patches. The adhesive properties of the patch have a direct correlation with drug permeation and flux notably for matrix system patches. For potent therapeutics, the quality, adhesion strength and minor tampering during wear can significantly impact patch’s in vivo performance. Adhesive performance can fluctuate over the duration of wear. Poor cohesive strength of the adhesive can lead to the formation of a 'dark ring' on the skin, which diminishes contact between the skin and the adhesive film, resulting in suboptimal drug absorption, particularly for patches worn over extended periods. Subtle changes in polymer interactions can markedly affect adhesion, with storage-induced three-dimensional changes potentially influencing adhesive performance and drug characteristics. Despite these complexities, no compendial test currently exists for measuring adhesive strength in transdermal patches. The necessity for standardized and reliable methods to assess adhesive performance is critical to ensuring consistent quality and therapeutic efficacy of transdermal patches. In this current investigation, besides the conventional probe-tack test, and the in vitro permeation test, the feasibility of using interferometry for studying the surface topography, and infra-red thermography for assessing the structural and adhesive deficiencies of transdermal patches was developed and validated.
Methods: In this study, surface topography was characterized using a Zygo NewView 600 interferometer, and roughness values were computed using Gwyddion software. Thermal properties of the patches were assessed via thermal analysis using a Fluke Ti480 Pro Infrared Camera, analyzing both fresh and expired patches with results processed using FLIR Thermal Studio software. The feasibility of these techniques to assess adhesive behavior was validated by tampering both fresh and expired patches across different tampering rounds (2R, 5R, 10R). The adhesiveness of transdermal patches was investigated using a probe-tack test, where a probe contacts the surface and is then retracted at a defined speed. The force required to break the bond after a short period of contact is plotted in a force–time diagram. Permeation profiles of nicotine patches (both intact and tampered) were evaluated using vertical Franz diffusion cells with a receiver orifice-area of 1.77 cm². The diffusion medium consisted of PBS with 2% ethanol, maintained at 37 °C and pH 6. Excised porcine ear explants of uniform thickness were employed in the permeation studies. Tampering experiments involved manually removing adhesive material from the patches using acetone-soaked cotton swabs, with tampering conducted for fixed rounds and duration. Validity and uniformity of the tampering technique were confirmed by measuring patch thickness pre- and post-tampering with a digital vernier calipers, quantifying the removed drug, and analyzing patch roughness and topography via interferometry after multiple tampering rounds. Statistical analysis utilized one-way ANOVA and Tukey’s post-hoc test to detect significant differences between datasets.
Results: Interferometry showed significant roughness differences between un-tampered and tampered patches (p < 0.05, 95% CI) among test patches. Specifically, 2R and 10R tampered sets differed significantly. No significant differences were found among reference patches (p >0.05, 95% CI), which was confirmed by roughness profiles in Figure 1A. Tampered est patches exhibited higher peaks, suggesting greater loss of adhesive than reference patches, indicating superior quality of the adhesive used in the manufacturing of reference patches. In Figure 1B, all patches exhibited a temperature difference of over 2°C compared to the external maximum temperature, indicating prolonged equilibration times for the test patches. Tampering in test patches showed a minor heat distribution improvement in un-expired patches but a significant enhancement in expired patches, suggesting accelerated adhesive deterioration post-expiration. This deterioration is likely due to the adhesive's polymeric properties, which reduces heat conduction. In contrast, reference patches consistently demonstrated superior heat distribution across all tampering levels. Adhesive performance evaluation by probe-tack test (Figure 2A) indicated a significant difference in the yield points of reference (-6N) and test patches (-1N). This difference could be attributed to the difference in quality of the adhesive used in manufacturing these both brands of patches. The flux values obtained from the in vitro permeation test of reference, test, ref-tampered, and test-tamp were 84.121±12.9, 10.79±18.14, 168.93±7.82, and 32.91±9.62 µg/cm2/h respectively (Figure 2B), which proves the superior performance of the reference patches.
Conclusion: Transdermal patch performance was assessed via interferometry for roughness, infrared spectroscopy for thermal imaging, probe-tack test for adhesiveness, and IVPT for drug permeation across porcine skin. Reference patches demonstrated superior adhesive conductivity and uniform distribution compared to test patches. The data demonstrate the feasibility of thermal imaging and interferometry as adjunct techniques to differentiate the adhesive performance of Q2 equivalent transdermal patches manufactured differently. The above comprehensive series of tests offers valuable insights to industry and academia in developing generic transdermal patches.
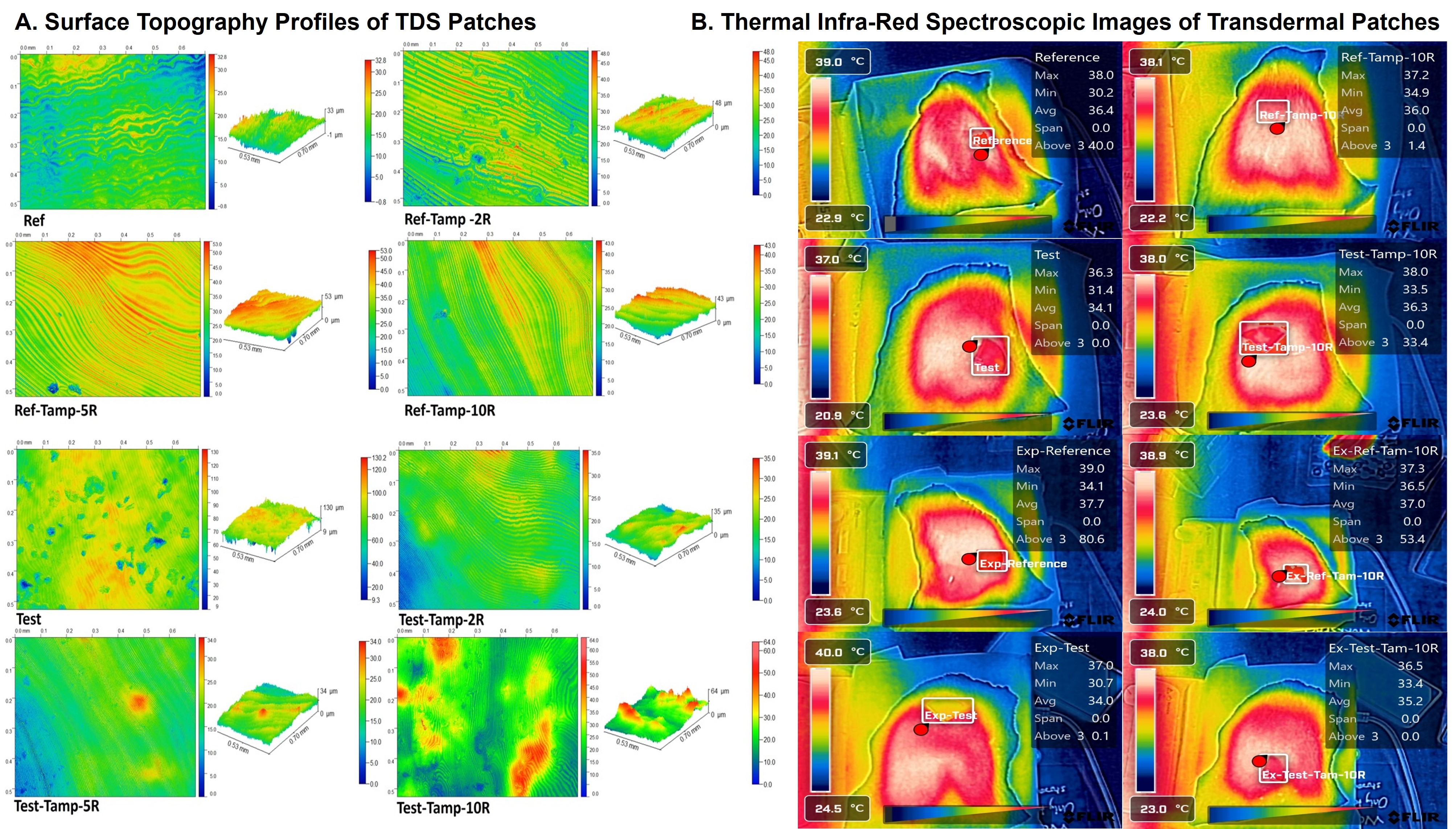 Figure 1: Surface topography, roughness profiles, and thermal infra-red spectroscopic images of transdermal patches. (A). Surface topography, and roughness profiles of Reference and Test patches. Un-expired, 2R, 5R, and 10R tampered patches were tested (n=3; p=0.0093, 95% CI). (B). Infra-red thermal imaging was conducted for the same sets of variables. The mean difference in the surface temperature of the skin where the patch was adhered was recorded (n=3). The images were captured when the temperature was at least above 37±1 °C.
Figure 1: Surface topography, roughness profiles, and thermal infra-red spectroscopic images of transdermal patches. (A). Surface topography, and roughness profiles of Reference and Test patches. Un-expired, 2R, 5R, and 10R tampered patches were tested (n=3; p=0.0093, 95% CI). (B). Infra-red thermal imaging was conducted for the same sets of variables. The mean difference in the surface temperature of the skin where the patch was adhered was recorded (n=3). The images were captured when the temperature was at least above 37±1 °C.
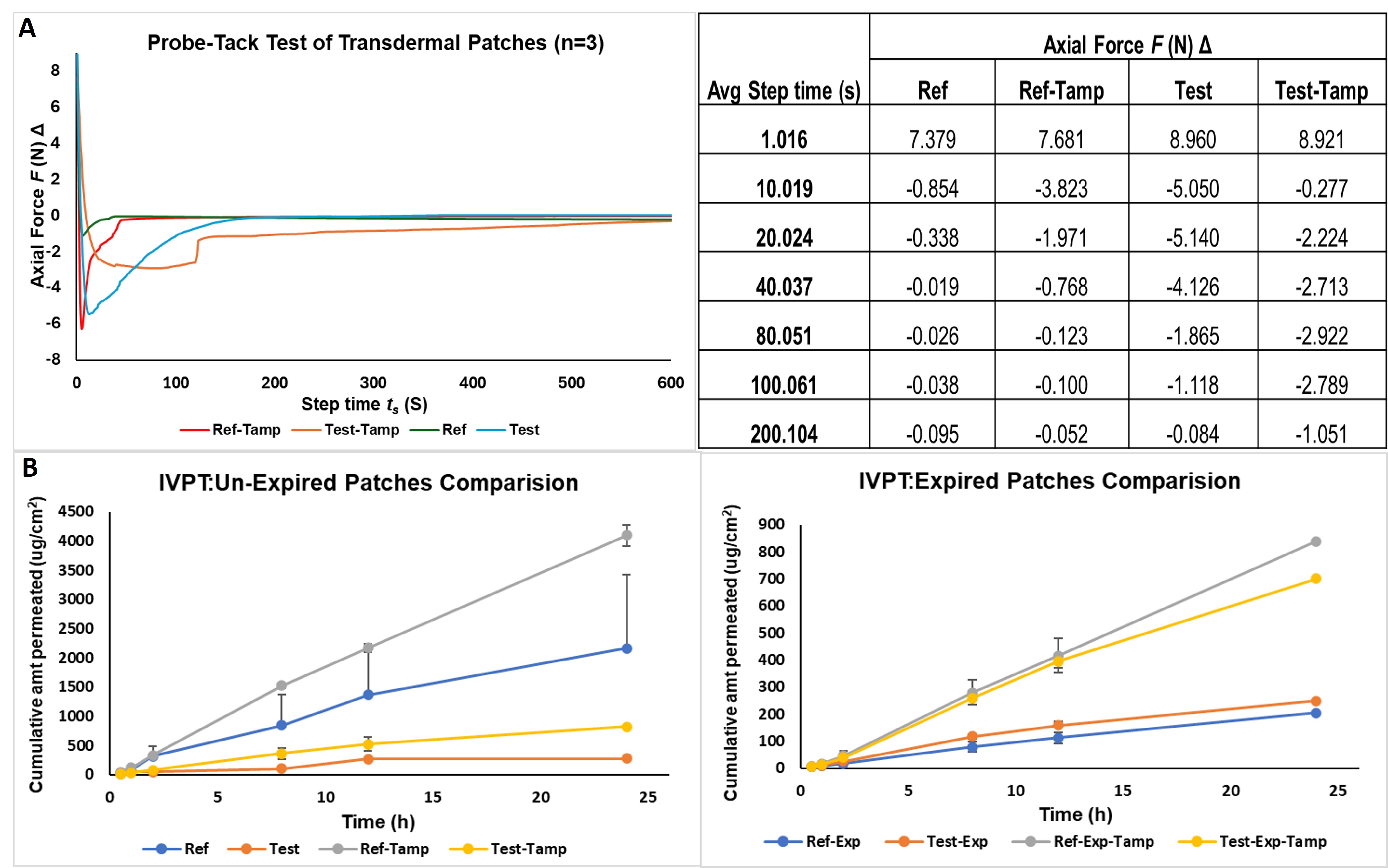 Figure 2: Adhesiveness performance evaluation and in vitro drug permeation testing of transdermal patches. (A). Adhesiveness performance was evaluated by probe-tack test using a TA instruments DHR-2 Rheometer. A 8 mm stainless steel peltier plate, with a diameter of 40 mm, loading gap of 30 mm was maintained. An axial force of 10N was applied for 30 seconds, and the experiment was conducted at 25 °C (n=3) (B). In vitro permeation testing of the reference and test transdermal patches were conducted using a vertical Franz diffusion cell of 1.77 cm2 receiver cell orifice area. Porcine ear explant was used, and the experiments were conducted at 37±0.5°C (n=3, p<0.5).
Figure 2: Adhesiveness performance evaluation and in vitro drug permeation testing of transdermal patches. (A). Adhesiveness performance was evaluated by probe-tack test using a TA instruments DHR-2 Rheometer. A 8 mm stainless steel peltier plate, with a diameter of 40 mm, loading gap of 30 mm was maintained. An axial force of 10N was applied for 30 seconds, and the experiment was conducted at 25 °C (n=3) (B). In vitro permeation testing of the reference and test transdermal patches were conducted using a vertical Franz diffusion cell of 1.77 cm2 receiver cell orifice area. Porcine ear explant was used, and the experiments were conducted at 37±0.5°C (n=3, p<0.5).
Methods: In this study, surface topography was characterized using a Zygo NewView 600 interferometer, and roughness values were computed using Gwyddion software. Thermal properties of the patches were assessed via thermal analysis using a Fluke Ti480 Pro Infrared Camera, analyzing both fresh and expired patches with results processed using FLIR Thermal Studio software. The feasibility of these techniques to assess adhesive behavior was validated by tampering both fresh and expired patches across different tampering rounds (2R, 5R, 10R). The adhesiveness of transdermal patches was investigated using a probe-tack test, where a probe contacts the surface and is then retracted at a defined speed. The force required to break the bond after a short period of contact is plotted in a force–time diagram. Permeation profiles of nicotine patches (both intact and tampered) were evaluated using vertical Franz diffusion cells with a receiver orifice-area of 1.77 cm². The diffusion medium consisted of PBS with 2% ethanol, maintained at 37 °C and pH 6. Excised porcine ear explants of uniform thickness were employed in the permeation studies. Tampering experiments involved manually removing adhesive material from the patches using acetone-soaked cotton swabs, with tampering conducted for fixed rounds and duration. Validity and uniformity of the tampering technique were confirmed by measuring patch thickness pre- and post-tampering with a digital vernier calipers, quantifying the removed drug, and analyzing patch roughness and topography via interferometry after multiple tampering rounds. Statistical analysis utilized one-way ANOVA and Tukey’s post-hoc test to detect significant differences between datasets.
Results: Interferometry showed significant roughness differences between un-tampered and tampered patches (p < 0.05, 95% CI) among test patches. Specifically, 2R and 10R tampered sets differed significantly. No significant differences were found among reference patches (p >0.05, 95% CI), which was confirmed by roughness profiles in Figure 1A. Tampered est patches exhibited higher peaks, suggesting greater loss of adhesive than reference patches, indicating superior quality of the adhesive used in the manufacturing of reference patches. In Figure 1B, all patches exhibited a temperature difference of over 2°C compared to the external maximum temperature, indicating prolonged equilibration times for the test patches. Tampering in test patches showed a minor heat distribution improvement in un-expired patches but a significant enhancement in expired patches, suggesting accelerated adhesive deterioration post-expiration. This deterioration is likely due to the adhesive's polymeric properties, which reduces heat conduction. In contrast, reference patches consistently demonstrated superior heat distribution across all tampering levels. Adhesive performance evaluation by probe-tack test (Figure 2A) indicated a significant difference in the yield points of reference (-6N) and test patches (-1N). This difference could be attributed to the difference in quality of the adhesive used in manufacturing these both brands of patches. The flux values obtained from the in vitro permeation test of reference, test, ref-tampered, and test-tamp were 84.121±12.9, 10.79±18.14, 168.93±7.82, and 32.91±9.62 µg/cm2/h respectively (Figure 2B), which proves the superior performance of the reference patches.
Conclusion: Transdermal patch performance was assessed via interferometry for roughness, infrared spectroscopy for thermal imaging, probe-tack test for adhesiveness, and IVPT for drug permeation across porcine skin. Reference patches demonstrated superior adhesive conductivity and uniform distribution compared to test patches. The data demonstrate the feasibility of thermal imaging and interferometry as adjunct techniques to differentiate the adhesive performance of Q2 equivalent transdermal patches manufactured differently. The above comprehensive series of tests offers valuable insights to industry and academia in developing generic transdermal patches.
 Figure 1: Surface topography, roughness profiles, and thermal infra-red spectroscopic images of transdermal patches. (A). Surface topography, and roughness profiles of Reference and Test patches. Un-expired, 2R, 5R, and 10R tampered patches were tested (n=3; p=0.0093, 95% CI). (B). Infra-red thermal imaging was conducted for the same sets of variables. The mean difference in the surface temperature of the skin where the patch was adhered was recorded (n=3). The images were captured when the temperature was at least above 37±1 °C.
Figure 1: Surface topography, roughness profiles, and thermal infra-red spectroscopic images of transdermal patches. (A). Surface topography, and roughness profiles of Reference and Test patches. Un-expired, 2R, 5R, and 10R tampered patches were tested (n=3; p=0.0093, 95% CI). (B). Infra-red thermal imaging was conducted for the same sets of variables. The mean difference in the surface temperature of the skin where the patch was adhered was recorded (n=3). The images were captured when the temperature was at least above 37±1 °C.  Figure 2: Adhesiveness performance evaluation and in vitro drug permeation testing of transdermal patches. (A). Adhesiveness performance was evaluated by probe-tack test using a TA instruments DHR-2 Rheometer. A 8 mm stainless steel peltier plate, with a diameter of 40 mm, loading gap of 30 mm was maintained. An axial force of 10N was applied for 30 seconds, and the experiment was conducted at 25 °C (n=3) (B). In vitro permeation testing of the reference and test transdermal patches were conducted using a vertical Franz diffusion cell of 1.77 cm2 receiver cell orifice area. Porcine ear explant was used, and the experiments were conducted at 37±0.5°C (n=3, p<0.5).
Figure 2: Adhesiveness performance evaluation and in vitro drug permeation testing of transdermal patches. (A). Adhesiveness performance was evaluated by probe-tack test using a TA instruments DHR-2 Rheometer. A 8 mm stainless steel peltier plate, with a diameter of 40 mm, loading gap of 30 mm was maintained. An axial force of 10N was applied for 30 seconds, and the experiment was conducted at 25 °C (n=3) (B). In vitro permeation testing of the reference and test transdermal patches were conducted using a vertical Franz diffusion cell of 1.77 cm2 receiver cell orifice area. Porcine ear explant was used, and the experiments were conducted at 37±0.5°C (n=3, p<0.5). 