Bioanalytics - Biomolecular
(M1430-06-31) A Sensitive Bioanalysis Method for Antisense Oligonucleotides using LC-MS/MS
Monday, October 21, 2024
2:30 PM - 3:30 PM MT
- LB
Lee Betram, BS
LCMS Scientist
Agilent Technologies, Inc
Santa Clara, California, United States - LB
Lee Betram, BS
LCMS Scientist
Agilent Technologies, Inc
Santa Clara, California, United States
Presenting Author(s)
Main Author(s)
Purpose: The purpose of this experiment was to design a sensitive bioanalysis method utilizing an automatable sample preparation method combined with the high sensitivity and robustness of the 6495D triple quadrupole mass spectrometer for a 2’-O-2-methoxyethyl, fully phosphorothioated oligonucleotide in human plasma samples.
Methods: A fully phosphorothioated single stranded oligonucleotide with the sequence U*/i2MOErC/*/i2MOErA/* /i2MOErC/*U*U* U*/i2MOErC/*/i2MOErA/* U*/i2MOErA/*/i2MOErA/* U*/i2MOErG/*C* U*/i2MOErG/*G was purchased from IDT. The calibration curve was prepared by spiking the oligonucleotide into extracted human plasma. The human plasma was extracted using a mixed mode weak anion exchange resin SPE plate and an automated liquid handler. Samples were run on a UPLC with a triple quadrupole mass spectrometer.
The LC method used a mobile phase A consisting of 15mM DIEA & 100mM HFIP in water with a mobile phase B of 100% methanol in a gradient starting with 20% B that ramped to 90% B over 6.5-minutes. The mass spectrometer was run in negative ion mode with a scan type of MRM. The MRM transitions monitored the -6 (905.9 m/z) and -7 (1057.0 m/z) charge states of the ASO.
Results: The results show that ASOs could be quantified with high confidence as low as 0.07 ng/mL in a plasma matrix. Excellent response, S/N of 153, and reproducibility of 0.06% RSD of the chosen ASO was shown at the lowest concentration in an extracted plasma matrix demonstrates the sensitivity of a LC-TQ system. Chromatographic stability was also assessed for all injections and a consistent retention time was shown across all concentrations and replicates with a retention time % RSD of 0.003. With minor adjustments to the LC metho gradient you can ensure separation and accurate quantitation of a variety of ASOs.
Conclusion: The method shown provides an end-to-end solution for low detection limits of quantitation for ASOs in human plasma by combining sample preparation via SPE on an automated liquid handler combined with a standard flow liquid chromatogram coupled with an LC/TQ. The strategy applied in this experiment can be applied easily and adapted to varying oligonucleotides in complex matrices.
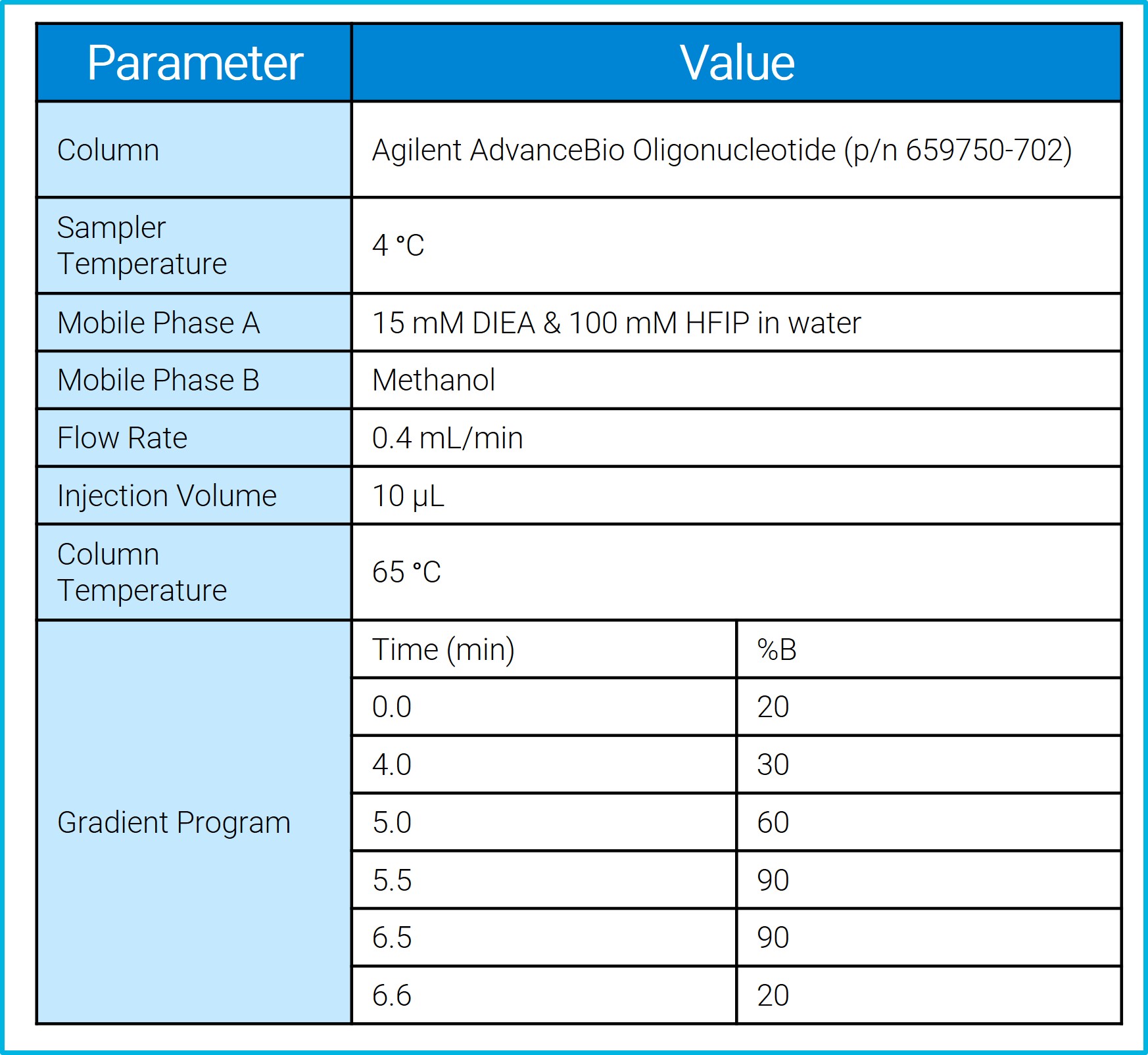 LC Method Table
LC Method Table
.jpg) Table including results for each of the standard injections. Results are the average of three replicate injections.
Table including results for each of the standard injections. Results are the average of three replicate injections.
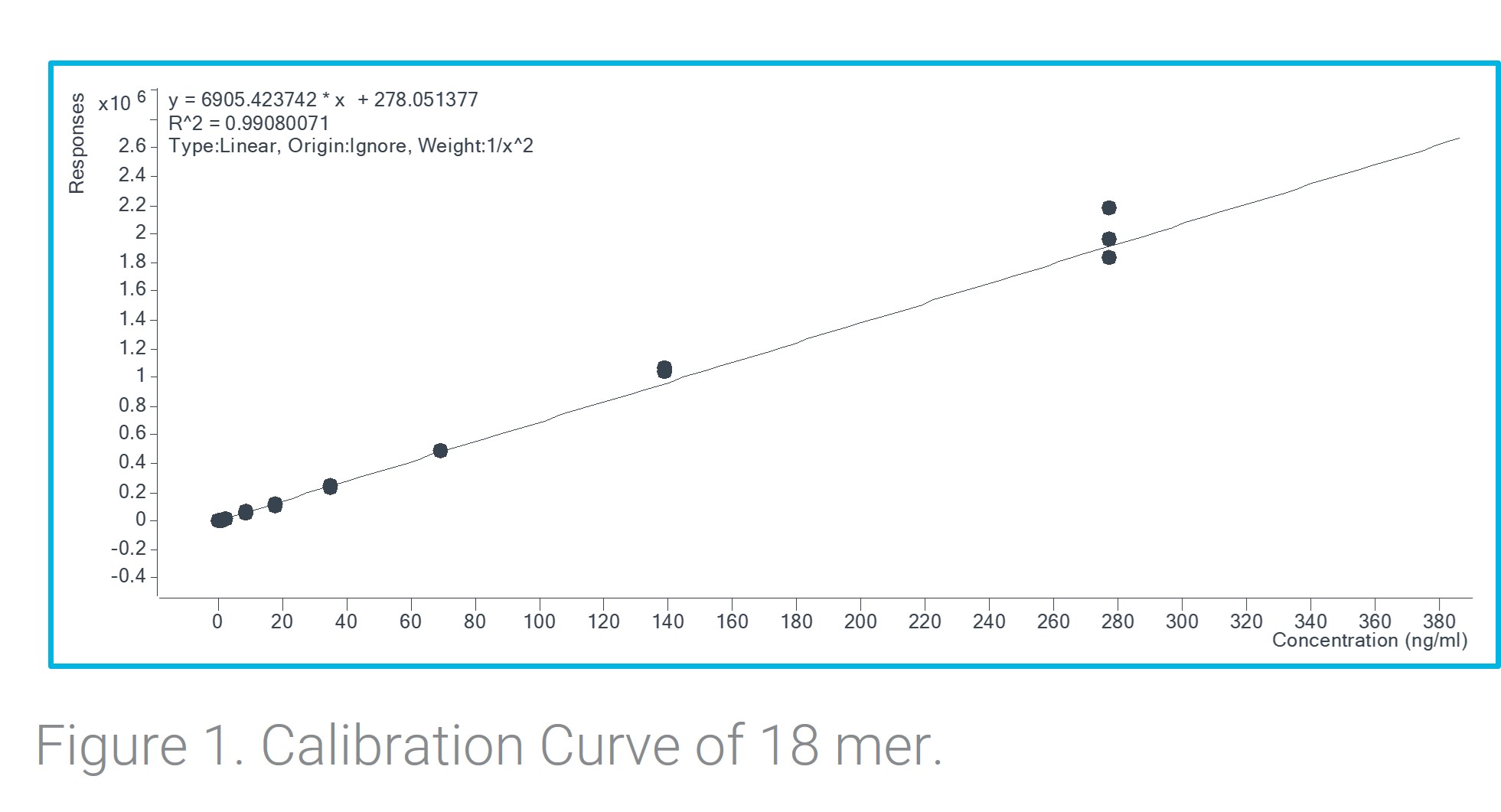 Figure showing calibration curve of the ASO
Figure showing calibration curve of the ASO
Methods: A fully phosphorothioated single stranded oligonucleotide with the sequence U*/i2MOErC/*/i2MOErA/* /i2MOErC/*U*U* U*/i2MOErC/*/i2MOErA/* U*/i2MOErA/*/i2MOErA/* U*/i2MOErG/*C* U*/i2MOErG/*G was purchased from IDT. The calibration curve was prepared by spiking the oligonucleotide into extracted human plasma. The human plasma was extracted using a mixed mode weak anion exchange resin SPE plate and an automated liquid handler. Samples were run on a UPLC with a triple quadrupole mass spectrometer.
The LC method used a mobile phase A consisting of 15mM DIEA & 100mM HFIP in water with a mobile phase B of 100% methanol in a gradient starting with 20% B that ramped to 90% B over 6.5-minutes. The mass spectrometer was run in negative ion mode with a scan type of MRM. The MRM transitions monitored the -6 (905.9 m/z) and -7 (1057.0 m/z) charge states of the ASO.
Results: The results show that ASOs could be quantified with high confidence as low as 0.07 ng/mL in a plasma matrix. Excellent response, S/N of 153, and reproducibility of 0.06% RSD of the chosen ASO was shown at the lowest concentration in an extracted plasma matrix demonstrates the sensitivity of a LC-TQ system. Chromatographic stability was also assessed for all injections and a consistent retention time was shown across all concentrations and replicates with a retention time % RSD of 0.003. With minor adjustments to the LC metho gradient you can ensure separation and accurate quantitation of a variety of ASOs.
Conclusion: The method shown provides an end-to-end solution for low detection limits of quantitation for ASOs in human plasma by combining sample preparation via SPE on an automated liquid handler combined with a standard flow liquid chromatogram coupled with an LC/TQ. The strategy applied in this experiment can be applied easily and adapted to varying oligonucleotides in complex matrices.
 LC Method Table
LC Method Table.jpg) Table including results for each of the standard injections. Results are the average of three replicate injections.
Table including results for each of the standard injections. Results are the average of three replicate injections. Figure showing calibration curve of the ASO
Figure showing calibration curve of the ASO 