Manufacturing and Analytical Characterization - Chemical
(M1430-10-55) Formulation Development of High Drug Loaded, Stable Amorphous Solid Dispersions (ASDs) Through Spray Drying: An Industrial Perspective Case Study
Monday, October 21, 2024
2:30 PM - 3:30 PM MT
- ND
Nagireddy Dumpa, Ph.D.
Sr. Scientist
SRI International
Sunnyvale, California, United States - ND
Nagireddy Dumpa, Ph.D.
Sr. Scientist
SRI International
Sunnyvale, California, United States - ML
Mingtao Liu, Ph.D.
Sr. Scientist
SRI International
menlo park, California, United States - NP
Nicky Pham, BS
Associate Technician
SRI international
menlo park, California, United States - JW
Jennie Wang, Ph.D.
Director
SRI International
menlo park, California, United States - GS
Gita Shankar, Ph.D.
Sr. Director
SRI Internatioanl
menlo park, California, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: The purpose of this study is to formulate and manufacture an ASD formulation at high drug loading into a capsule for a poorly soluble API using spray drying technique.
Methods: A lab scale Büchi Mini Spray Dryer B-290 was used for the spray drying process. Total of six polymers (PVP-VA 64, Soluplus®, Eudragit L100, HPMCP-HP55, HPMCAS-M and HPMC) at two different drug loadings (20% and 40%w/w) were investigated. API and each polymer were dissolved in suitable solvent at 40:60 and 20:80 w/w drug to polymer ratios (the quantity of solids dissolved in solvent was kept at 5% w/w) and the solution is used for spray drying. Spray Dried Dispersions (SDDs) obtained were test in-vivo in rats and placed on stability at both 25C°/60%RH and 45°C/75%RH conditions. From the rat in-vivo study and the stability data, HPMC formulation at 40% drug loading was selected for the manufacturing of high-drug loaded capsules. SDD powder obtained with HPMC polymer showed low bulk density and poor flow properties. Roller compaction process was added to the capsule manufacturing step to increase the bulk density and improve the flow properties of the SDD powder. Spray drying and roller compaction formulation composition and process parameters were provided in Figure 1. Excipient compatibility studies were conducted to select the suitable excipients for the capsule formulation. Roller compacted granules were blended with suitable excipients selected from the excipient compatibility study and filled into size “0” HPMC capsules at 100.0 mg strength. Capsule formulation blend was tested in-vivo in rats to assess effect of the roller compaction and formulation composition on the bioavailability. Manufactured capsules were tested for assay, PXRD, water content, residual solvent and dissolution performance and placed on stability study at both 25C°/60%RH and 45°C/75%RH conditions.
Results: From the six polymers investigated, HPMC polymer demonstrated better stability and bioavailability compared to other five polymers and was selected for manufacturing of 100 mg strength capsules. Pure SDD powder showed bioavailability of 70.1±4.7% (n=3) in male rats and 123±50.4% (n=3) in female rats. Capsule blend demonstrated bioavailability of 72±4.88 % (n=3) in male rats and 125±12% (n=3) in female rats. Rat plasma concentrations of API are provided in Figure 2A and 2B. Roller compaction increased the bulk density of the SDD powder by more than 120%. Capsules filled with the formulation blend showed more than 85% drug release in 60.0 minutes (Figure 3A) meeting the criteria of immediate release dosage forms. During spray drying process crystalline API is converted into amorphous form and remained in amorphous form during roller compaction process (Figure 3B). Analytical testing results of manufactured 100.0 mg capsules are presented in Figure 3. Finalized capsule formulations were stable up to three months at both 25C°/60%RH and 45°C/75%RH) stability conditions.
Conclusion: ASD formulation of poorly soluble drug was successfully developed with HPMC polymer at 40% drug loading using spray drying technique with enhanced bioavailability. Roller compaction process was developed and optimized to increase the bulk density and flow properties of the SDD material there by improving manufacturing efficiency and reducing the pill burden without affecting the dissolution rate and bioavailability. In conclusion, a high drug loaded enabled stable ASD capsule formulation was developed and manufactured.
Acknowledgements: This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Oregon Health and Science University's Prime Contract No.75N93023C00002.
The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the U.S. Government.
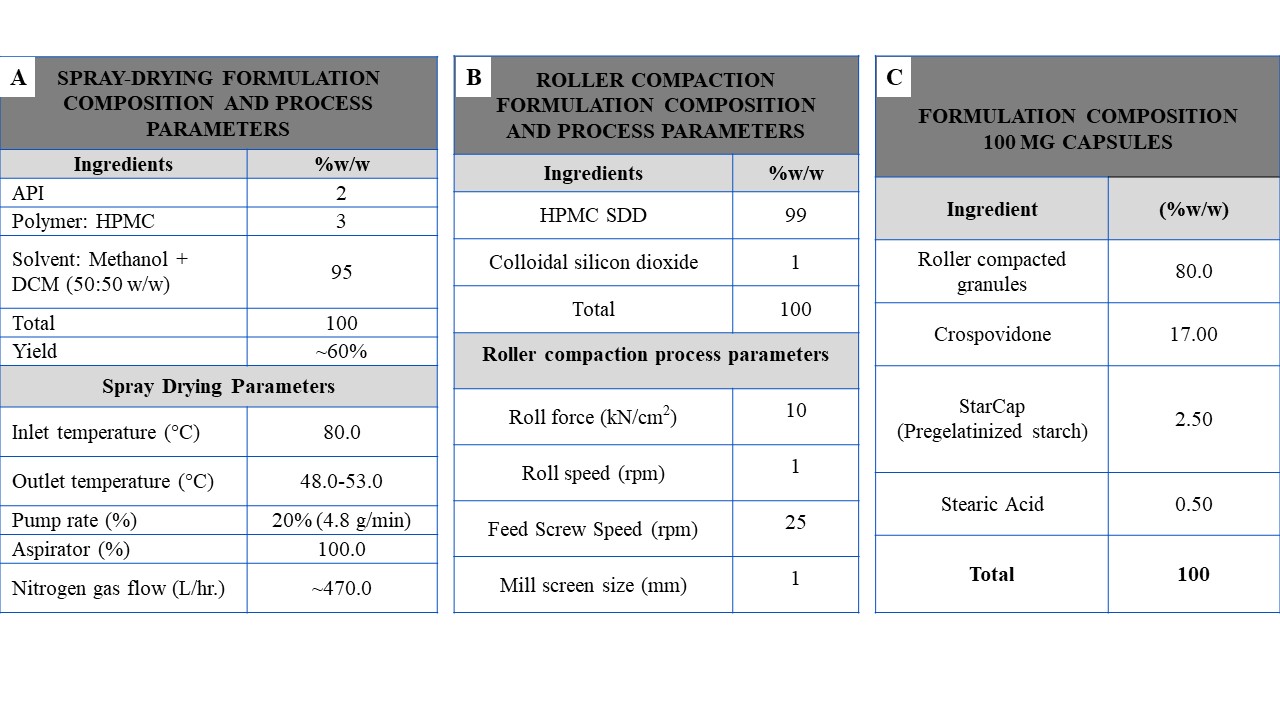 Figure 1. (A)Spray drying formulation composition and process parameters (B) Roller compaction formulation composition and process parameters (c) Formulation composition of 100 mg capsules
Figure 1. (A)Spray drying formulation composition and process parameters (B) Roller compaction formulation composition and process parameters (c) Formulation composition of 100 mg capsules
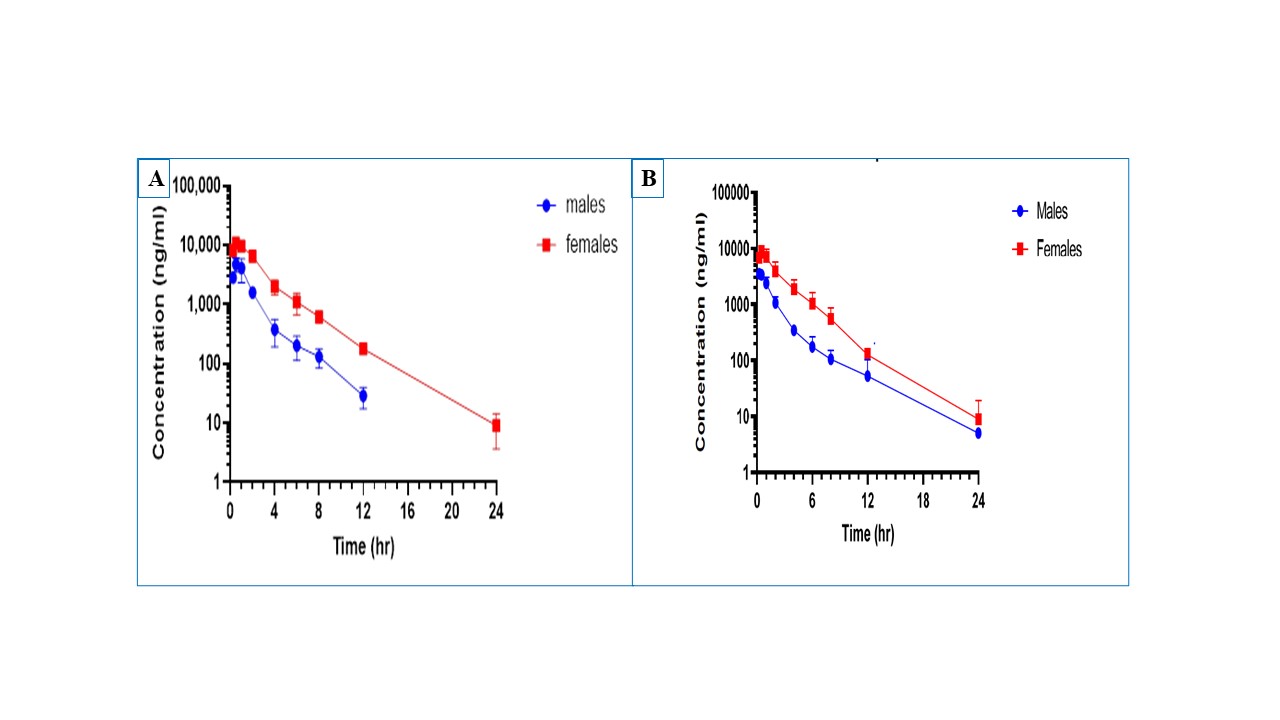 Figure 2. (A) Plasma profiles of API after PO administration of pure SDD powder (A) and formulated capsule blend (B) at 25 mg/kg dose to male and female Sprague Dawley rats
Figure 2. (A) Plasma profiles of API after PO administration of pure SDD powder (A) and formulated capsule blend (B) at 25 mg/kg dose to male and female Sprague Dawley rats
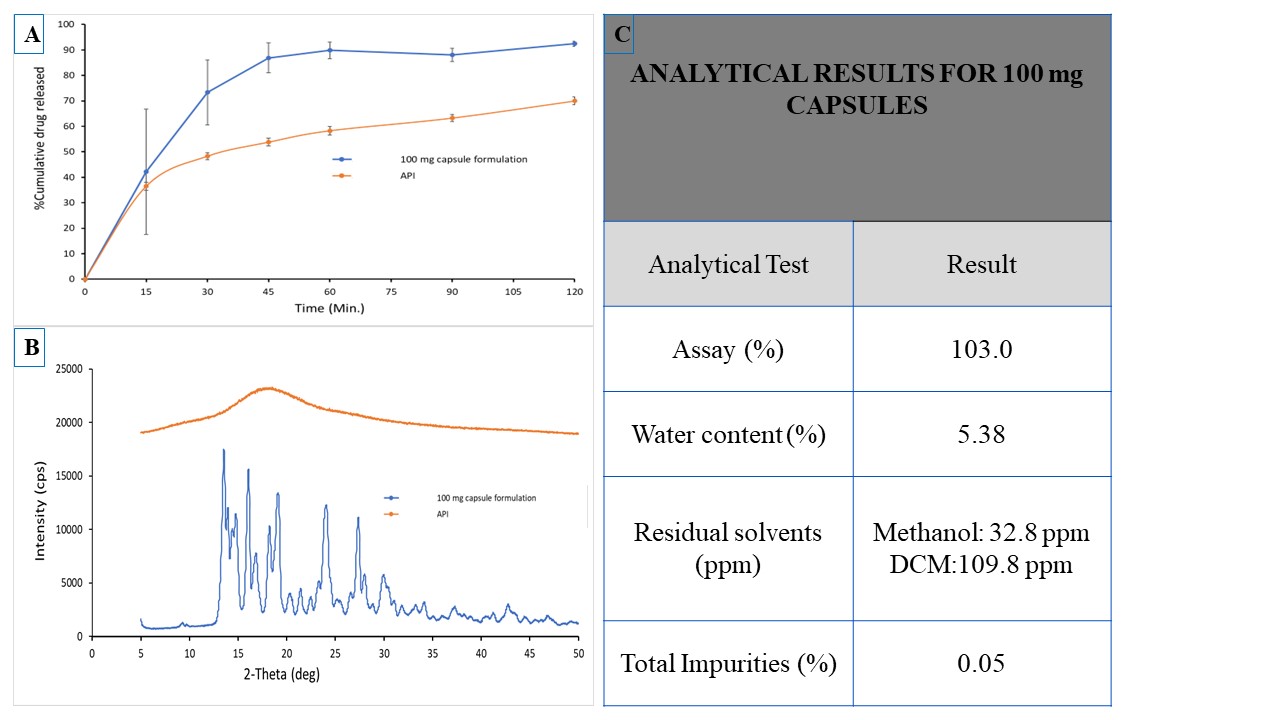 Figure 3. (A) Dissolution profile of pure API and formulated 100 mg capsule (B) PXRD diffractograms of Pure API and formulated 100 mg capsule (C) Analytical testing results of 100 mg capsules
Figure 3. (A) Dissolution profile of pure API and formulated 100 mg capsule (B) PXRD diffractograms of Pure API and formulated 100 mg capsule (C) Analytical testing results of 100 mg capsules
Methods: A lab scale Büchi Mini Spray Dryer B-290 was used for the spray drying process. Total of six polymers (PVP-VA 64, Soluplus®, Eudragit L100, HPMCP-HP55, HPMCAS-M and HPMC) at two different drug loadings (20% and 40%w/w) were investigated. API and each polymer were dissolved in suitable solvent at 40:60 and 20:80 w/w drug to polymer ratios (the quantity of solids dissolved in solvent was kept at 5% w/w) and the solution is used for spray drying. Spray Dried Dispersions (SDDs) obtained were test in-vivo in rats and placed on stability at both 25C°/60%RH and 45°C/75%RH conditions. From the rat in-vivo study and the stability data, HPMC formulation at 40% drug loading was selected for the manufacturing of high-drug loaded capsules. SDD powder obtained with HPMC polymer showed low bulk density and poor flow properties. Roller compaction process was added to the capsule manufacturing step to increase the bulk density and improve the flow properties of the SDD powder. Spray drying and roller compaction formulation composition and process parameters were provided in Figure 1. Excipient compatibility studies were conducted to select the suitable excipients for the capsule formulation. Roller compacted granules were blended with suitable excipients selected from the excipient compatibility study and filled into size “0” HPMC capsules at 100.0 mg strength. Capsule formulation blend was tested in-vivo in rats to assess effect of the roller compaction and formulation composition on the bioavailability. Manufactured capsules were tested for assay, PXRD, water content, residual solvent and dissolution performance and placed on stability study at both 25C°/60%RH and 45°C/75%RH conditions.
Results: From the six polymers investigated, HPMC polymer demonstrated better stability and bioavailability compared to other five polymers and was selected for manufacturing of 100 mg strength capsules. Pure SDD powder showed bioavailability of 70.1±4.7% (n=3) in male rats and 123±50.4% (n=3) in female rats. Capsule blend demonstrated bioavailability of 72±4.88 % (n=3) in male rats and 125±12% (n=3) in female rats. Rat plasma concentrations of API are provided in Figure 2A and 2B. Roller compaction increased the bulk density of the SDD powder by more than 120%. Capsules filled with the formulation blend showed more than 85% drug release in 60.0 minutes (Figure 3A) meeting the criteria of immediate release dosage forms. During spray drying process crystalline API is converted into amorphous form and remained in amorphous form during roller compaction process (Figure 3B). Analytical testing results of manufactured 100.0 mg capsules are presented in Figure 3. Finalized capsule formulations were stable up to three months at both 25C°/60%RH and 45°C/75%RH) stability conditions.
Conclusion: ASD formulation of poorly soluble drug was successfully developed with HPMC polymer at 40% drug loading using spray drying technique with enhanced bioavailability. Roller compaction process was developed and optimized to increase the bulk density and flow properties of the SDD material there by improving manufacturing efficiency and reducing the pill burden without affecting the dissolution rate and bioavailability. In conclusion, a high drug loaded enabled stable ASD capsule formulation was developed and manufactured.
Acknowledgements: This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Oregon Health and Science University's Prime Contract No.75N93023C00002.
The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the U.S. Government.
 Figure 1. (A)Spray drying formulation composition and process parameters (B) Roller compaction formulation composition and process parameters (c) Formulation composition of 100 mg capsules
Figure 1. (A)Spray drying formulation composition and process parameters (B) Roller compaction formulation composition and process parameters (c) Formulation composition of 100 mg capsules Figure 2. (A) Plasma profiles of API after PO administration of pure SDD powder (A) and formulated capsule blend (B) at 25 mg/kg dose to male and female Sprague Dawley rats
Figure 2. (A) Plasma profiles of API after PO administration of pure SDD powder (A) and formulated capsule blend (B) at 25 mg/kg dose to male and female Sprague Dawley rats  Figure 3. (A) Dissolution profile of pure API and formulated 100 mg capsule (B) PXRD diffractograms of Pure API and formulated 100 mg capsule (C) Analytical testing results of 100 mg capsules
Figure 3. (A) Dissolution profile of pure API and formulated 100 mg capsule (B) PXRD diffractograms of Pure API and formulated 100 mg capsule (C) Analytical testing results of 100 mg capsules