Preclinical, Clinical, and Translational Sciences
(M1530-02-07) Evaluation of the Pharmacokinetics and Abuse Potential of Intranasally Administered Extended-Release Oxycodone and Naloxone Abuse-Deterrent Formulations Milled to Different Particle Sizes

Manar Al-Ghabeish, PhD (she/her/hers)
Senior Pharmacologist
US Food and Drug Administration
Silver Spring, Maryland, United States
Manar Al-Ghabeish, PhD (she/her/hers)
Senior Pharmacologist
US Food and Drug Administration
Silver Spring, Maryland, United States- MK
Minori Kinjo, Ph.D.
Senior Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States 
Heather J. Boyce, Ph.D.
LEAD PHARMACOKINETICIST
US Food and Drug Administration
Silver Spring, Maryland, United States- JO
John Oldenhof, Ph.D.
Executive Vice President, Scientific Affairs
BioPharma Services Inc.
North York, Ontario, Canada - SR
Sofia Raitsin, Ph.D.
Sr. Clinical Pharmacology Scientist
Biopharma Services Inc.
Toronto, Ontario, Canada - MK
Myong-Jin Kim, Pharm.D.
Division Director
US Food and Drug Administration
Silver Spring, Maryland, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: The study was a single-dose, randomized, double-blind, active controlled, five-way crossover study. The study subjects were non-dependent recreational opioid users who underwent a naloxone challenge and discrimination test to ensure they could discriminate between the positive drug effects of the active control and placebo, and also tolerate the study products. The subjects were randomly assigned to receive one of these five treatments in five consecutive treatment periods: three different manipulated products of oxycodone hydrochloride (HCl) 40 mg; naloxone HCl 20 mg extended-release (ER) tablets (Treatment A: fine [106–300 µm] particles, Treatment B: medium [300–600 µm] particles, and Treatment C: coarse [600–1000 µm] particles), milled oxycodone HCl immediate-release tablets (Treatment D: active control, oxycodone HCl 40 mg dose), and placebo (Treatment E: microcrystalline cellulose powder). All treatments were administered nasally via insufflation using a 0.20-inch diameter plastic straw. PK plasma samples were obtained at 15 timepoints and analyzed for oxycodone, oxymorphone, and naloxone. The primary PK parameters of interest were the maximum observed plasma concentration (Cmax) and area under the plasma concentration vs. time curve (AUCs). The PD assessment included ease of snorting, Drug liking (including visual analog scale [VAS] maximum effect [Emax], Take Drug Again, pupillometry and Subject-Rated Assessment of Intranasal Irritation (SRAII). In addition, safety endpoints included adverse events, vital signs, lab test results, physical and nasal cavity examination.
Results: A total of 40 subjects, which included 11 females and 29 males, were randomized to the Treatment Phase and received at least one dose of study drug. Crushing oxycodone HCl; naloxone HCl ER tabletimpaired the ER mechanism of the dosage form, and the insufflation of crushed products resulted in the rapid release and absorption of oxycodone, oxymorphone and naloxone. For oxycodone and oxymorphone, the rates and extents (except of early extent) of absorption were comparable between the three manipulated products despite different particle size ranges (90% confidence intervals of Cmax and AUCinf were within acceptance range of 80.00-120.00 %, Figure 1. As particle size increased (particle size of A < B < C), the extent of absorption at the early stage (pAUC0-0.5, 0-1 and 0-2) tended to decrease. Treatment D showed a distinct PK profile with a double peak observed for all subjects. This phenomenon has been reported previously for oxycodone. [1] For naloxone, a lower rate and extent of absorption were observed as the particle size increased (Figure 1). The PD assessment of the study was validated by the significant difference of Emax for Drug Liking between the active control and placebo (p < 0.001).Overall, the three different manipulated products demonstrated statistically significant AD potential compared to the active control (p < 0.001). For example, at least 50% of subjects showed at least 30 % reduction in Drug Liking VAS Emax for the manipulated products compared to the active control. The results also indicate comparable AD potential between the manipulated products with different particle sizes. All study treatments were well tolerated without unexpected or serious adverse events. The most common adverse events ( >10% of subjects) reported in the treatment phase were somnolence (50.0%), headache (30.0%), euphoric mood (15.0%), hypoxia (12.5%), nausea (12.5%), agitation (10.0%), pruritus (10.0%) and hypertension (10.0%). These adverse events are commonly associated with treatment with opioids.
Conclusion:
Intranasal administration of the manipulated products with smaller particles resulted in a faster and greater extent of exposure for naloxone but not for oxycodone and its active metabolite, oxymorphone. However, the change in the nasal bioavailability of naloxone did not impact the AD potential of the product as measured by Drug Liking. The AD potential of this product via the nasal route was not impacted by the particle size of the insufflated product.
References: 1.Clinical Pharmacology and Biopharmaceutics Review, NDA 205777, Orig1s000, available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205777Orig1s000ClinPharmR.pdf accessed on: 6/26/2024
Acknowledgements: Disclaimer: This work reflects the views of the authors and should not be construed to represent FDA’s views or policies.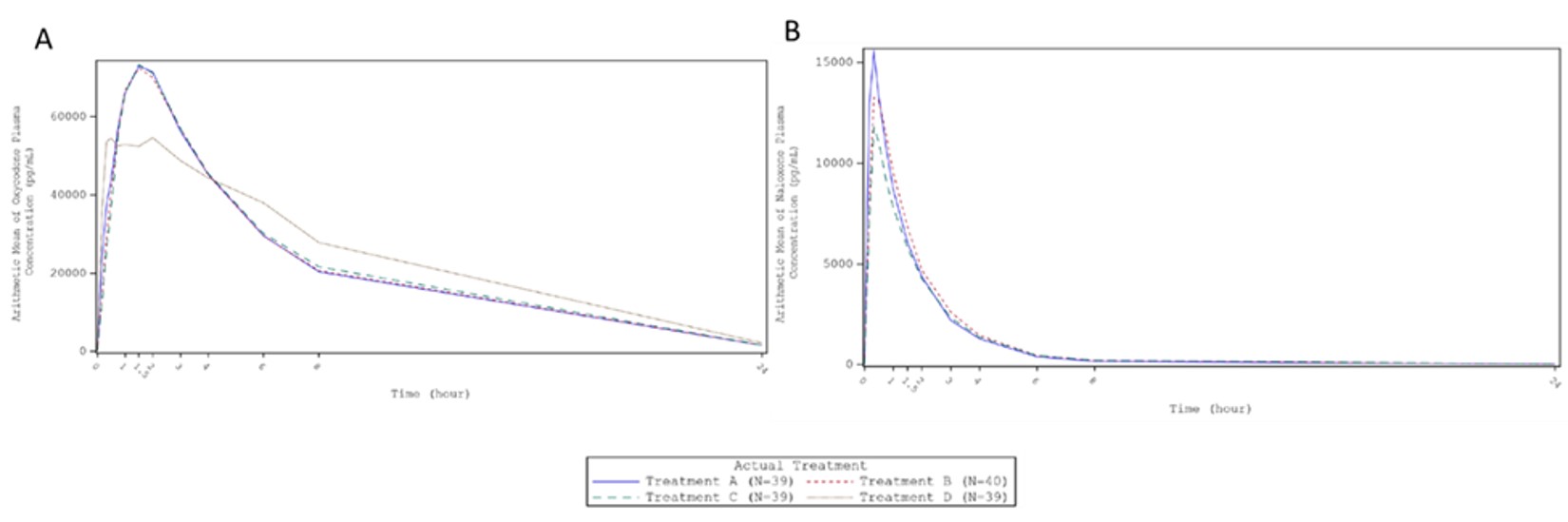 Figure 1: Mean Plasma Concentration vs Time Profiles for Oxycodone (A) and Naloxone (B) after Intranasal Administration of Treatments A,B,C, and D
Figure 1: Mean Plasma Concentration vs Time Profiles for Oxycodone (A) and Naloxone (B) after Intranasal Administration of Treatments A,B,C, and D
