Formulation and Delivery - Biomolecular
(M1330-07-37) Immunoactive Nanoparticle Boosted cDC1 for In Situ Cancer Immunotherapy

Chih-Jia Chao, Pharm.D. (she/her/hers)
Student
University of Illinois Chicago
Chicago, Illinois, United States
Chih-Jia Chao, Pharm.D. (she/her/hers)
Student
University of Illinois Chicago
Chicago, Illinois, United States- EZ
Endong Zhang, Ph.D.
Post doc
University of Illinois Chicago
Chicago, Illinois, United States - DT
Duong N. Trinh, Ph.D.
Post doc
University of Illinois Chicago
Chicago, Illinois, United States - SH
Shan He, Ph.D.
Post doc
University of Illinois Chicago
Chicago, Illinois, United States - JZ
Jingtian Zheng, Ph.D.
Post doc
University of Illinois Chicago
Chicago, Illinois, United States - QB
Qing Bao, Ph.D.
Post doc
University of Illinois Chicago
Chicago, Illinois, United States - PP
Philana Phan, BS
PhD student
University of Illinois Chicago
Chicago, Illinois, United States - SE
Sara M Elgendy, BS
PhD student
University of Illinois Chicago
Chicago, Illinois, United States - XS
Xiangqian Shi, BS
PhD student
University of Illinois Chicago
Chicago, Illinois, United States - SL
Steve Seung Young Lee, Ph.D.
Assistant Professor in Pharmaceutical Sciences
University of Illinois Chicago
Chicago, Illinois, United States - YG
Yu Gao, Ph.D.
Associate Professor
University of Illinois Chicago
Chicago, Illinois, United States 
Zongmin Zhao, Ph.D.
Assistant Professor
University of Illinois Chicago
Chicago, Illinois, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: We employ a Trojan horse strategy leveraging antigen capturing nanoparticles (AC-NPs) and migratory CD103+ type 1 conventional dendritic cells (cDC1s), named Antigen Capturing nanoparticle Transformed Dendritic Cell therapy (ACT-DC). AC-NPs are engineered to capture antigens directly from the tumor and facilitate their delivery to adoptively transferred migratory CD103+ cDC1s, which enhance antigen presentation to the immune cells in lymph nodes and reshape the tumor microenvironment.
Results: Our findings show that ACT-DC possesses robust antigen-capturing capability and dendritic cell activation ability. AC-NP captured significantly more tumor associated proteins compared to that of control nanoparticles (Fig. 1a-b), and induced significantly higher expression of activation markers on cDC1 (Fig. 1c-d). Due to the efficient migration ability of cDC1, ACT-DC increased the quantity of injected cDC1s in tDLNs (Fig. 1e). To prove antigen transport by ACT-DC, AF647-labeled ovalbumin (OVA) were intratumorally injected as a model tumor antigen. ACT-DC resulted in a 2-4.9-fold increase in the number of injected cDC1s carrying OVA compared to the injection of cDC1s alone or in combination with control NPs (Fig 1f). By transporting in situ captured tumor antigens to tumor-draining lymph nodes (tDLNs), ACT-DC is designed to initiate a cascade systemic antitumor immune response and modulate tumor environment. To evaluate this mechanism, we measured the immune cell profiles in the tDLNs and tumors of mice treated with ACT-DC. ACT-DC resulted in a significant increase in the number of T cells (Fig. 2a), antigen-specific CD8 T cells (Fig. 2b), and memory T cells in tDLNs (Fig. 2c). In the tumors, ACT-DC significantly increased the infiltration of CD4 and CD8 T cells, including effector CD8 T cells and Th1 cells compared to the PBS treatment (Fig. 2d-e). Our findings showed that ACT-DC could trigger a strong systemic immune response with memory effects and transform the tumor environment into a more immunogenic state. To evaluate the therapeutic efficacy of ACT-DC, we monitored tumor sizes and survival in mice with MC38 and B16F10 tumors. Notably, 40-80% of mice treated with ACT-DC achieved complete tumor eradication and survived without detectable tumor recurrence on day 80 post-primary tumor inoculation in both tumor models. With the synergic effects of ACT-DC and anti-PD1 antibody, tumor-free survival reached 75-100% (Fig. 3a-b and Fig. 3e-f). To assess the immune memory generated by ACT-DC, we rechallenged the surviving tumor-free mice. ACT-DC, either alone or in combination with aPD1, demonstrated significantly superior efficacy in inhibiting the growth of the rechallenged tumors (Fig. 3c-d and Fig. 3g-h).
Conclusion: In this work, we highlight the capability of ACT-DC to enhance in situ immunization for systemic tumor eradication. ACT-DC harness intratumoral antigen, modulates local tumor microenvironment, and improves antigen presentation leading to efficient anti-cancer immunity and long-term tumor rejection. The ACT-DC approach could be a broadly effective strategy for in situ cancer immunization and tumor microenvironment modulation.
References: 1. W Wang, H Xu, Q Ye, F Tao, I Wheeldon, et al. Nat. Biomed. Eng. 2022, 6, 44.
2. J Chen, M Qiu, Z Ye, T Nyallle, Y Li, et al. Sci. Adv. 2021, 7, eabf1244.
3. W Yang, G Zhu, S Wang, G Yu, Z Yang, et al. ACS Nano. 2019, 13, 3083.
Acknowledgements: Authors acknowledge funding from NIH (R35GM150507) and Vahlteich Award and Startup fund from the College of Pharmacy at the University of Illinois Chicago.
.jpg) Figure 1. ACT-DC improved antigen presentation with AC-NPs and migratory cDC1s. (a) Tumor protein binding capability of AC-NPs. (b) Number of unique proteins captured by AC-NPs from tumor lysate. c-d, Expression of DC activation markers, including (c) CD80 and (d) CD86. (e) IVIS images of injected CD103+ cDC1 in tDLNs 6 or 20 hours after intratumoral injection of ACT-DC. (f) Percentage of activated AF647-OVA-carrying, adoptively transferred CD103+ cDC1 in tDLNs, 20 hrs after treatment
Figure 1. ACT-DC improved antigen presentation with AC-NPs and migratory cDC1s. (a) Tumor protein binding capability of AC-NPs. (b) Number of unique proteins captured by AC-NPs from tumor lysate. c-d, Expression of DC activation markers, including (c) CD80 and (d) CD86. (e) IVIS images of injected CD103+ cDC1 in tDLNs 6 or 20 hours after intratumoral injection of ACT-DC. (f) Percentage of activated AF647-OVA-carrying, adoptively transferred CD103+ cDC1 in tDLNs, 20 hrs after treatment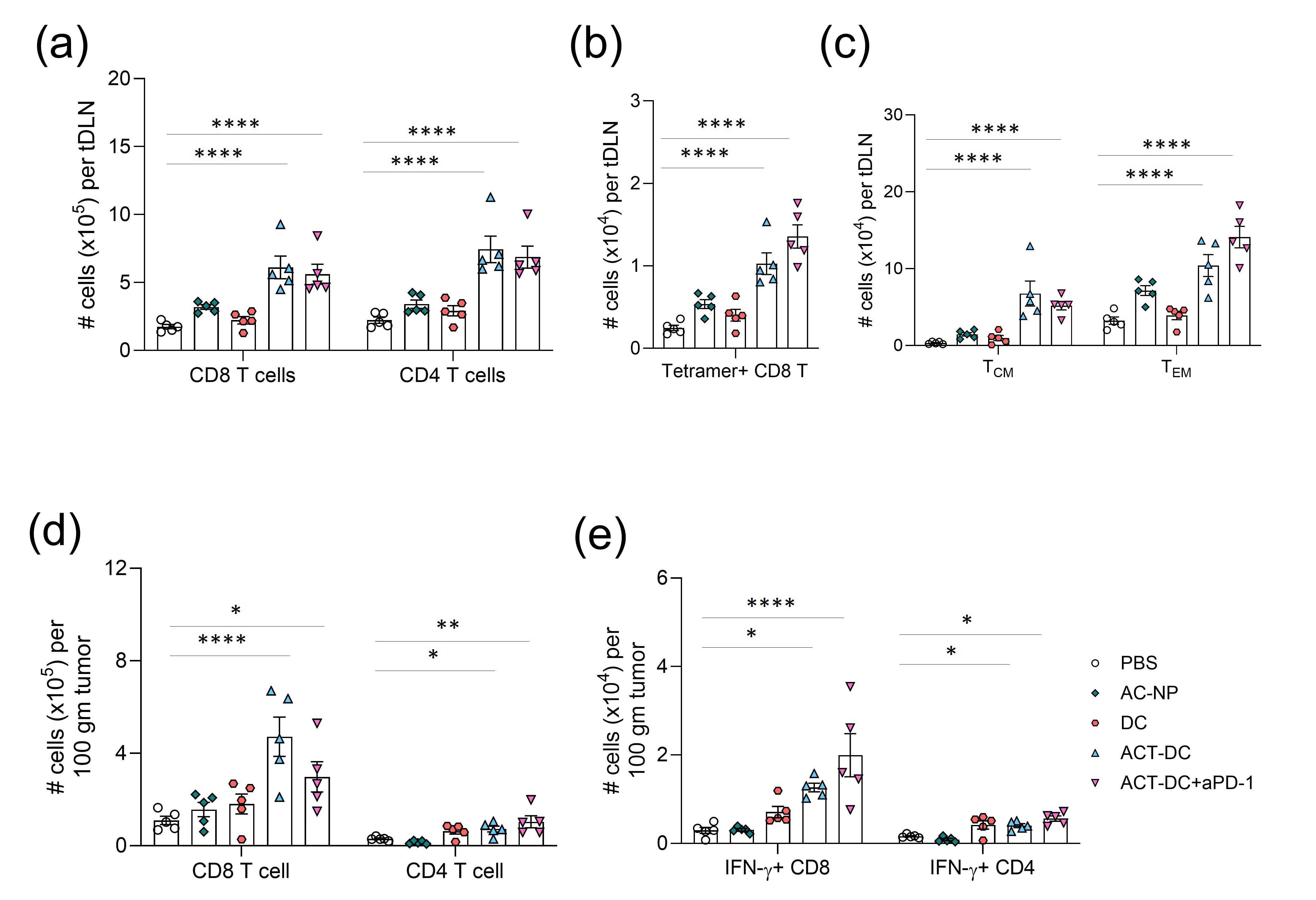 Figure 2. Immune cell profiles in tDLN and tumor after ACT-DC therapy. a-c, Number of immune cells in the tDLN including (a) CD8/CD4 T cells (b) tetramer positive CD8 T cells and (c) memory CD8 T cells. d-e, Number of immune cell in tumors, including (d) CD8 /CD4 T cells and (e) IFN-γ-expressing CD8/CD4 T cells.
Figure 2. Immune cell profiles in tDLN and tumor after ACT-DC therapy. a-c, Number of immune cells in the tDLN including (a) CD8/CD4 T cells (b) tetramer positive CD8 T cells and (c) memory CD8 T cells. d-e, Number of immune cell in tumors, including (d) CD8 /CD4 T cells and (e) IFN-γ-expressing CD8/CD4 T cells.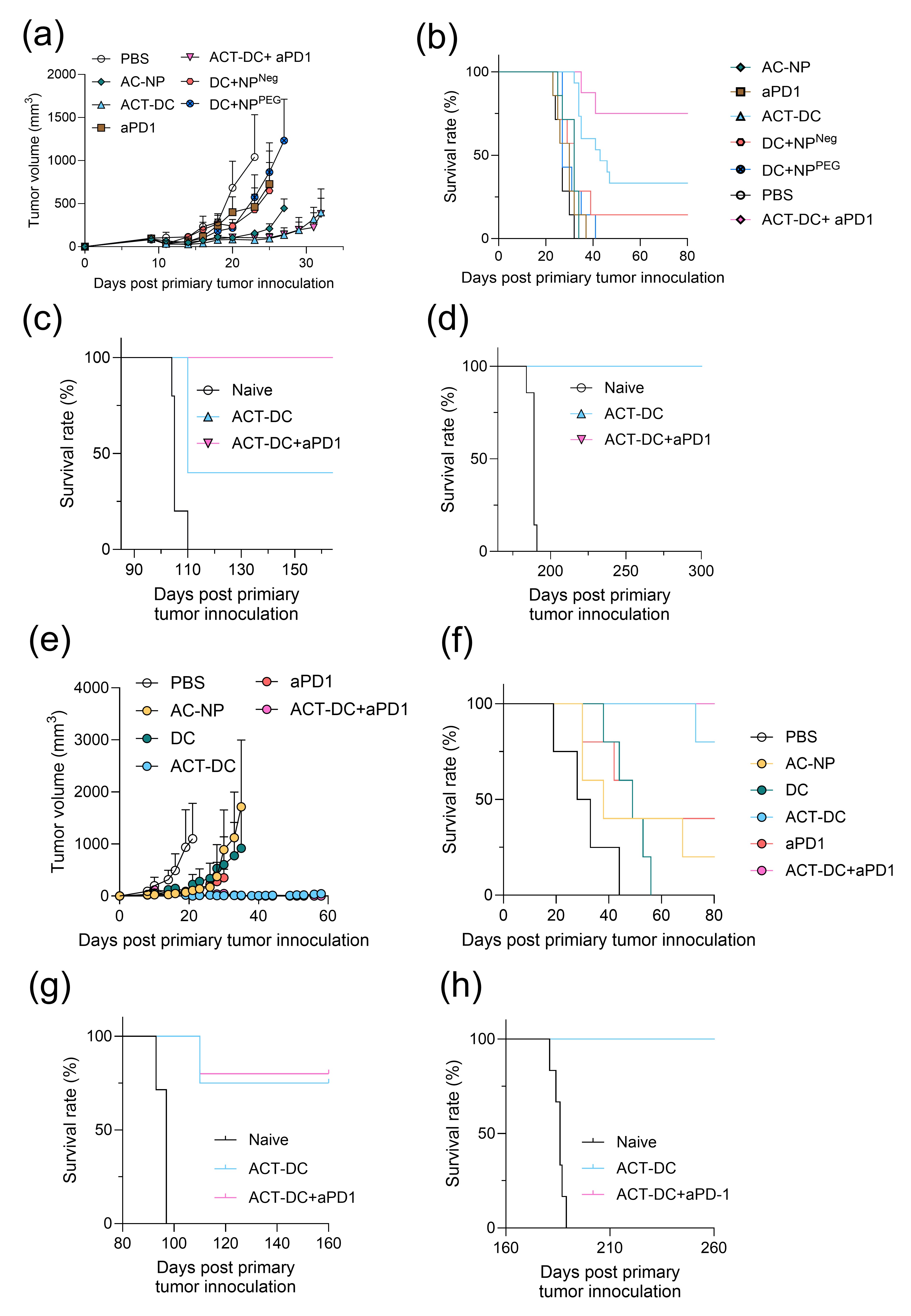 Figure 3. Evaluate the therapeutic efficacy and memory effects of ACT-DC in an MC38 and B16F10 tumor model. (a) Tumor volume and (b) survival for primary MC38 tumor. c-d, Survival curve of mice after the (c) 1st rechallenge and (d) 2nd rechallenge of MC38 tumor. e-f, Therapeutic efficacy in B16F10 mice including (e) tumor volume and (f) survival of primary tumors. (g) Efficacy of ACT-DC in controlling the 1st s.c. tumor rechallenge. (h) survival curve after 2nd i.v. rechallenge.
Figure 3. Evaluate the therapeutic efficacy and memory effects of ACT-DC in an MC38 and B16F10 tumor model. (a) Tumor volume and (b) survival for primary MC38 tumor. c-d, Survival curve of mice after the (c) 1st rechallenge and (d) 2nd rechallenge of MC38 tumor. e-f, Therapeutic efficacy in B16F10 mice including (e) tumor volume and (f) survival of primary tumors. (g) Efficacy of ACT-DC in controlling the 1st s.c. tumor rechallenge. (h) survival curve after 2nd i.v. rechallenge.