Formulation and Delivery - Biomolecular
(T1030-10-57) Dry Powders of a Rabies G Protein mRNA Vaccine Candidate Prepared by Thin-Film Freeze-Drying

Robert O. Williams, III, PhD (he/him/his)
Professor
The University of Texas at Austin
Austin, Texas, United States
Robert O. Williams, III, PhD (he/him/his)
Professor
The University of Texas at Austin
Austin, Texas, United States
Zhengrong Cui, Ph.D.
Professor
University of Texas at Austin
Austin, Texas, United States- HX
Haiyue Xu, Ph.D.
Postdoctoral Fellow
University of Texas at Austin
Austin, Texas, United States 
Roland Böttger, PhD
Senior Scientist
CureVac
Tubingen, Baden-Wurttemberg, Germany- KA
Khaled AboulFotouh, Ph.D.
Postdoctoral Fellow
University of Texas at Austin
Austin, Texas, United States - DO
Donald Owens, Ph.D.
Director
TFF Pharmaceuticals, Inc.
Fort Worth, Texas, United States - CC
Chris Cano, MBA
Business Development Advisor
TFF Pharmaceuticals, Inc.
Fort Worth, Texas, United States - PB
Patrick Baumhof, Ph.D.
Director
CureVac
Tübingen, Baden-Wurttemberg, Germany
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: LNPs were formulated with mRNA that encodes either Rabies G protein or luciferase (as a model mRNA), an ionizable lipid, a hydrophilic lipid, a phospholipid, and cholesterol. The mRNA-LNPs were suspended in a solution containing 150 mM of sucrose, 75 mM NaCl, and 10 mM of phosphate (pH 7.4) and then stored frozen at -80oC. Before the mRNA-LNPs were subject to TFFD, they were mixed with a solution containing various amounts of trehalose, leucine, and poloxamer 188 (P188). The mRNA-LNP suspensions were then thin-film frozen as previously described on a stainless-steel surface that was cryogenically precooled to about -70oC. The mRNA-LNPs were characterized before and after being subject to TFFD and reconstitution. The stability of the mRNA-LNPs in dry powders were monitored for one year when stored refrigerated (2-8oC), or at 25oC or 40oC. As a control, the mRNA-LNPs were stored as a frozen suspension at -80oC. Critical quality parameters determined include particle size and polydispersity index (PDI) using dynamic light scattering (DLS), mRNA encapsulation efficiency using a Ribogreen assay, mRNA integrity using high-performance liquid chromatography (HPLC), lipid concentration by HPLC, and mRNA activity by measuring protein expression after transfecting HeLa cells. Finally, the immunogenicity of the Rabies G mRNA vaccine candidate before and after being subject to TFFD and reconstitution was evaluated in BALB/c mice (6-8 weeks, n = 7, 0.25 mg per mouse) upon intramuscular injection (twice on days 1 and 21). Mice were euthanized on day 28; blood was collected to measure Rabies virus-neutralizing titer, and spleen was harvested to determine T cell proliferation activity.
Results: Initially, a luciferase (Luc) mRNA was used to prepare mRNA-LNPs. The nanoparticles were unilamellar spheres with no detectable blebs, based on cryogenic electron microscopic (Cryo-EM) images. Their particle size and PDI were 62 ± 2 nm and 0.23 ± 0.03, respectively. The mRNA encapsulation efficiency was over 95%. Thin-film freeze-dried CuV1 and CuV4 dry powders (TFF CuV1 and TFF CuV4) were chosen for additional studies as the mRNA integrity and encapsulation efficiency, lipid concentrations, as well as the polydispersity of the mRNA-LNPs upon reconstitution were maintained. In vitro cell transfection data also did not reveal any significant difference among the luciferase expression levels in cells treated with the original Luc mRNA-LNPs or Luc mRNA-LNPs reconstituted from the TFF CuV1 or CuV4 powders. The particle size of the Luc mRNA-LNPs witnessed an increase of 4-20 nm based on DLS data, although cryo-EM images did not show any apparent particle size increase or particle aggregations after the mRNA-LNPs were subject to TFFD and reconstitution. Data from a one-year storage stability study showed that when the TFF CuV1 or CuV4 Luc mRNA-LNP dry powders were stored at 2-8oC, the particle size and polydispersity of the mRNA-LNPs, the integrity and encapsulation efficiency of the mRNA, as well as the cell transfection efficiency of the mRNA-LNPs remained unchanged. At 25oC and 40oC, the Luc mRNA-LNPs were less stable. Based on the promising in vitro data with the Luc mRNA-LNPs, we then formulated Rabies G protein mRNA in the same LNPs and thin-film freeze-dried the resultant Rabies G mRNA-LNPs into TFF CuV1 and CuV4 powders. Upon confirming that the particle size of the mRNA-LNPs and the integrity and encapsulation efficiency of the mRNA were maintained after the mRNA-LNPs were subject to TFFD, the immunogenicity of the mRNA-LNPs reconstituted from the powders was evaluated in a mouse model and compared to that of the original mRNA-LNPs (reference LNP). Shown in Fig. 1 are the Rabies virus neutralizing titers in the blood samples of the immunized mice, which did not show any significant difference between mice immunized with the original mRNA-LNPs vs. those immunized with mRNA-LNPs reconstituted from the TFF CuV1 or CuV4 powder.
Conclusion: It is feasible to apply TFFD to develop the Rabies G mRNA vaccine candidate as a dry powder product that is stable when stored refrigerated.
Acknowledgements: This work was supported in part by TFF Pharmaceuticals, Inc. ZC and ROW report a conflict of interest with TFF Pharma.
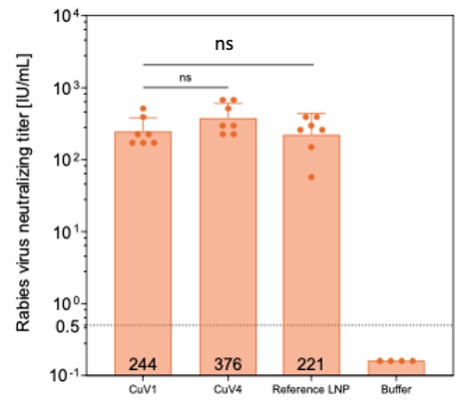 Figure 1. Rabies virus neutralizing titer in blood samples of mice intramuscularly immunized with Rabies G mRNA-LNPs, the original one as a reference or reconstituted from TFF CuV1 or CuV4 dry powders. Mice (n = 7) were immunized on days 1 and 21 and euthanized on day 28. The dose of mRNA was 0.25 mcg per mouse.
Figure 1. Rabies virus neutralizing titer in blood samples of mice intramuscularly immunized with Rabies G mRNA-LNPs, the original one as a reference or reconstituted from TFF CuV1 or CuV4 dry powders. Mice (n = 7) were immunized on days 1 and 21 and euthanized on day 28. The dose of mRNA was 0.25 mcg per mouse.