Formulation and Delivery - Chemical
(T1530-10-58) Optimizing Mucoadhesive Properties of Oral Thin Films: The Impact of Substrate Selection on Adhesive Force and Residence Time
Tuesday, October 22, 2024
3:30 PM - 4:30 PM MT
- RW
Renae Wilson, BSc, MS (she/her/hers)
PhD Candidate
Nova Southeastern University
Fort Lauderdale, Florida, United States - RW
Renae Wilson, BSc, MS (she/her/hers)
PhD Candidate
Nova Southeastern University
Fort Lauderdale, Florida, United States 
Sumana Dey Chowdhury, BSc, MS (she/her/hers)
PhD Candidate
Nova Southereastern University
Ft Lauderdale, Florida, United States- AA
Arnavaz Akhzamehr, MS, PharmD
PhD Student
Nova Southeastern University
Fort Lauderdale, Florida, United States - EG
Erma Gill, MS, PharmD
PhD Student
Nova Southeastern University
Davie, Florida, United States - HO
Hossein Omidian, Ph.D.
Professor
Nova Southeastern University
Fort Lauderdale, Florida, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Buccal drug delivery systems such as oral thin films (OTF) are non-invasive, offering numerous benefits including higher patient compliance and drug availability, and the ability to bypass the first pass-effect (1-4). The use of polymers enhances the adhesive properties and residence time which increases drug delivery via the buccal mucosa (3,4). Researchers have established a variety of methods with the use of biological substrates, such as animal tissues to assess the mucoadhesive properties of drug delivery systems, however, there are currently no USP-NF standardized methods for the characterization of oral thin films (6,7). This study investigates the adhesive properties and residence times of oral films (Vitamin B12, Ziminta, Listerine Red and Listerine Green) formulated with different film-forming polymers, including pullulan, sodium alginate, and hypromellose (HPMC). Using a texture analyzer, we measured the adhesive force of each film against various substrates and correlated these with their residence times. Our findings reveal significant differences in adhesive strength and retention, with sodium alginate exhibiting the highest adhesive force (AF) and longest residence time (RT). The Pearson correlation coefficient also indicated a strong positive correlation between the adhesive force and the residence time. This research provides crucial insights for optimizing polymer selection in oral film formulations to enhance performance and user experience.
Methods: Texture Analyzer Preparation: A texture analyzer CT3-1000 (Brookfield, MA, USA) was employed to determine AF. Parameters included a target force of 0.96 N, a hold time of 15 seconds, and a speed of 0.1 mm (about 0 in)/s, with 200 microliters (µl) of artificial saliva [10]. Oral thin film was attached to the probe with double-sided tape. Instrument force, distance, and speed were calibrated. The wax substrate was placed under the probe and 200 µl artificial saliva was used for conditioning purposes. The adhesive behavior (adhesive force) of four OTF formulations against various substrates was investigated with the use of the texture analyzer [10]. Glass slabs were coated with substrate and conditioned with 200 µl of artificial saliva. The OTF was placed on a glass slab. The prepared glass slab was then immersed vertically into a glass beaker containing 200 microliters of phosphate buffer (PBS) pH 6.8 at 37 °C ± 1 °C, followed by stirring at 60 rpm to simulate buccal conditions. Subsequently, the time required for the film to detach completely from the coated glass substrate was observed and recorded as the mucoadhesive retention time (RT). The experiment was conducted in triplicate, and the RT was determined based on the time taken for the film to fully detach from the substrate. [7, 11, 12, 13 & 14].
Results: The adhesive force (AF) was evaluated using three different substrates. PEG 1450 exhibited the lowest AF at 0.38 N for B12 and the highest at 1.036 N for Listerine Green. The adhesive force for Ziminta, B12, and Listerine Red in Cetostearyl alcohol was almost the same at 0.6 N, whereas for Listerine Green, it was slightly lower at 0.547 N. Beeswax yielded similar results for Ziminta and B12 at 1.3 N, with the lowest being Listerine Red at 0.8 N. Overall, Beeswax showed a higher mean AF compared to the other substrates. The residence time (RT) was evaluated using three different substrates. PEG 1450 showed the lowest RT of 0.4 minutes with Listerine Red and the highest of 12.88 minutes for B12. Cetostearyl alcohol ranged from 7.71 minutes (Ziminta) to 18.88 minutes (Listerine Green), indicating a moderate to high affinity for the films. However, residence times varied significantly, with Listerine Red showing the highest value of 33.09 minutes and Ziminta the lowest at 10.34 minutes, suggesting a high variability in film retention. Overall, the mean RT was highest for Beeswax and lowest for PEG 1450 when comparing the three substrates. The Pearson correlation coefficient was calculated and was found to be of approximately 0.888 indicates a strong positive correlation between AF and RT for the Ziminta oral film. For vitamin B12, Listerine Red, and Listerine Green, the results were found to be -0.236, -0.173, and -0.972 respectively. These results for the latter three oral thin films indicate that there is not a strong relationship between AF and RT for these specific films. Additionally, higher adhesive forces are strongly associated with shorter residence times for this film.
Conclusion: Beeswax exhibited the highest mean RT and AF, indicating strong retention on hydrophobic surfaces. Cetostearyl alcohol showed moderate residence times, suggesting its potential as an effective substrate. In contrast, PEG 1450 displayed the lowest RT and AF due to its high hydrophilicity. These findings underscore the importance of substrate selection in the development of buccal drug delivery systems. Hydrophobic substrates like Beeswax tend to enhance mucoadhesion, whereas hydrophilic substrates like PEG 1450 may reduce adhesive performance. Our in vitro method provides an alternative to biological substrates for assessing OTF properties.
References: 1. Pearce, A. K., & O’Reilly, R. K. (2021). Polymers for biomedical applications: The importance of hydrophobicity in directing biological interactions and application efficacy. Biomacromolecules, 22(11), 4459–4469. https://doi.org/10.1021/acs.biomac.1c00434
2. Hartig, T., Mohamed, A. T., Fattah, N. F., Gülses, A., Tjardts, T., Kangah, E. A., Chan, K. P., Veziroglu, S., Acil, Y., Aktas, O. C., Wiltfang, J., Loutfy, S. A., Strunskus, T., Faupel, F., Amin, A., & Schröder, S. (2023). ICVD polymer thin film bio‐interface‐performance for fibroblasts, cancer‐cells, and viruses connected to their functional groups and in silico studies. Advanced Materials Interfaces, 11(1). https://doi.org/10.1002/admi.202300587
3. Laffleur, F. (2014). Mucoadhesive polymers for buccal drug delivery. Drug Development and Industrial Pharmacy, 40(5), 591–598. https://doi.org/10.3109/03639045.2014.892959
4. Nazila Salamat-Miller, Montakarn Chittchang, Thomas P. Johnston. The use of mucoadhesive polymers in buccal drug delivery. Advanced Drug Delivery Reviews.Volume 57, Issue 11, 2005,Pages 1666-1691,ISSN 0169-409X, https://doi.org/10.1016/j.addr.2005.07.003.
5. Wang XQ, Iqbal J, Rahmat D, Bernkop-Schnurch A. Preactivated thiomers: permeation enhancing properties. Int J Pharm 2012;438:217–24
6. Bassi da Silva, J., Ferreira, S. B. de S., de Freitas, O., & Bruschi, M. L. (2017). A critical review of methodologies for the analysis of mucoadhesive properties of drug delivery systems. Drug Development and Industrial Pharmacy, 43(7), 1053–1070. https://doi.org/10.1080/03639045.2017.1294600
7. Alaei S, Omidian H. Mucoadhesion and Mechanical Assessment of Oral Films. Eur J Pharm Sci. 2021 Apr 1; 159: 105727. doi: 10.1016/j.ejps.2021.105727. Epub 2021 Jan 21. PMID: 33484813.
8. Kumar, A., Naik, P. K., Pradhan, D., Ghosh, G., & Rath, G. (2020). Mucoadhesive formulations: innovations, merits, drawbacks, and future outlook. Pharmaceutical Development and Technology, 25(7), 797–814. https://doi.org/10.1080/10837450.2020.1753771
9. Figueiras, A., Pais, A.A.C.C. & Veiga, F.J.B. A Comprehensive Development Strategy in Buccal Drug Delivery. AAPS PharmSciTech 11, 1703–1712 (2010). https://doi.org/10.1208/s12249-010-9546-1
10. Alaei, S., Omidi, Y., & Omidian, H. (2021). In vitro evaluation of adhesion and mechanical properties of oral thin films. European Journal of Pharmaceutical Sciences, 166, 105965.
11. Abruzzo, A., Nicoletta, F. P., Dalena, F., Cerchiara, T., Luppi, B., & Bigucci, F. (2017). Bilayered buccal films as child-appropriate dosage form for systemic administration of propranolol. International journal of pharmaceutics, 531(1), 257-265.
12. Ammar, H. O., Ghorab, M. M., Mahmoud, A. A., & Shahin, H. I. (2017). Design and in vitro/in vivo evaluation of ultra-thin mucoadhesive buccal film containing fluticasone propionate. Aaps Pharmscitech, 18, 93-103.
13. Fithri, A. N., Wijaya, D. P., & Taher, T. (2020). Optimization of chitosan–tapioca starch composite as polymer in the formulation of gingival mucoadhesive patch film for delivery of gambier (Uncaria gambir Roxb) leaf extract. International journal of biological macromolecules, 144, 289-295.
14. Mohamad, S. A., Sarhan, H. A., Abdelkader, H., & Mansour, H. F. (2017). Vitamin B12–loaded buccoadhesive films as a noninvasive supplement in vitamin B12 deficiency: In vitro evaluation and in vivo comparative study with intramuscular injection. Journal of pharmaceutical sciences, 106(7), 1849-1858.
Acknowledgements: Affiliation: - All authors are associated with the college of pharmacy at Nova Southeastern University in Fort Lauderdale, Florida.
Acknowledgements & Author contributions: - We acknowledge Nova Southeastern University facilities for the implementation of the research. HO, SDC, RLW, EJG and AA designed, analyzed, revised the data, and wrote the manuscript. SDC, RLW, EJG and AA conducted the experiments.
Disclaimer: - The statements, opinions and data contained in this abstract are solely those of the individual author(s) and contributor(s) and not of the AAPS, or the reviewer/editor. AAPS and/or the reviewer/editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Funding: - This research received no external funding.
Disclosure or conflict of interest statements: - The authors declare no conflicts of interest.
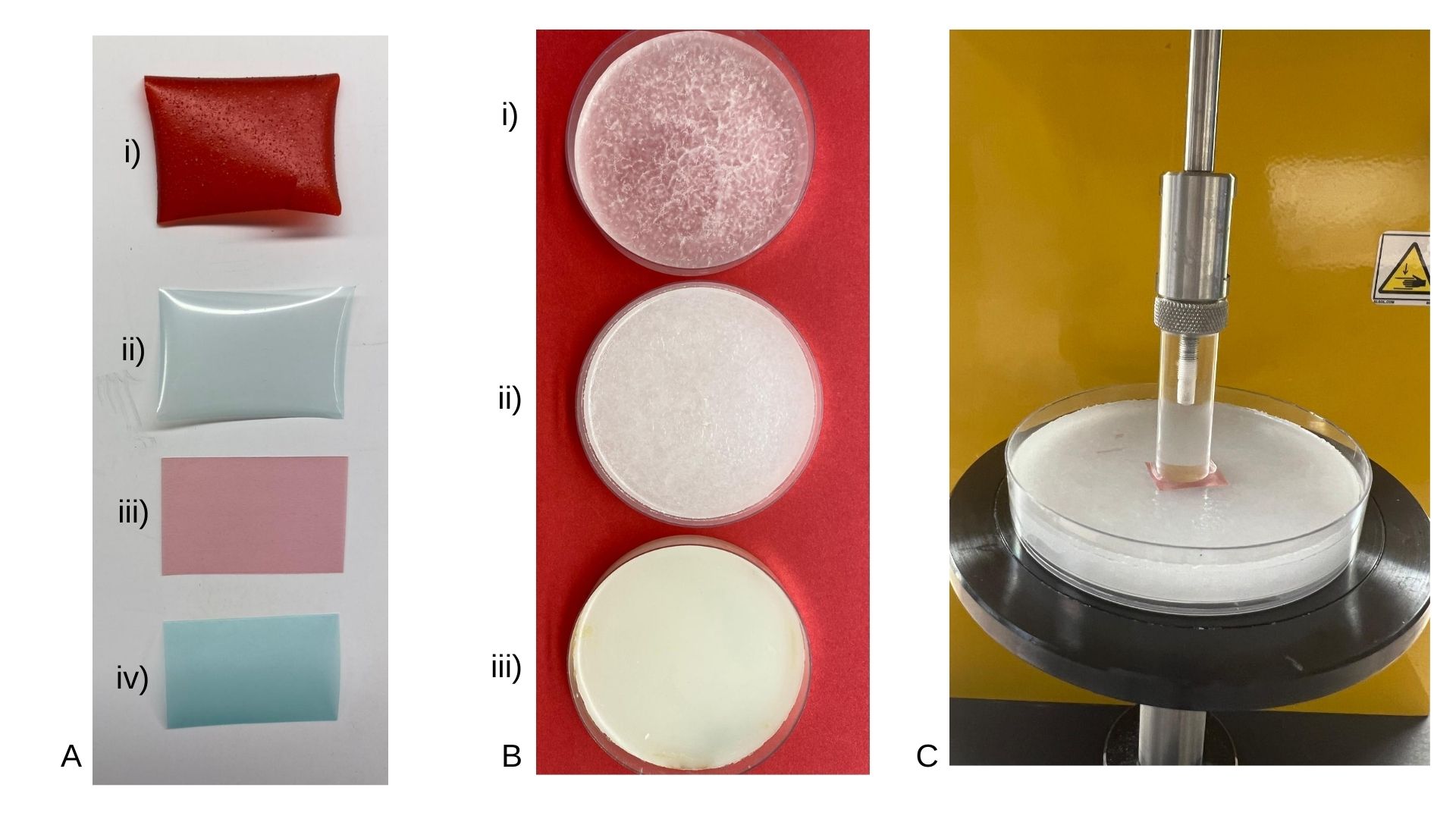 Figure 1. The mucoadhesion test set-up using the texture analyzer (TA) CT3-1000 (Brookfield, MA, USA). (A) Commercially available oral thin films: i) Vit B12 ii) Ziminta iii) Listerine Red and iv) Listerine Green (mint). (B) Substrates prepared in the lab using: i) PEG 1450 ii) Cetostearyl alcohol iii) Bees wax shown petri-dish (C) Represents TA probe, with double-sided adhesive tape, and Listerine red oral thin film assessed using Cetostearyl Alcohol.
Figure 1. The mucoadhesion test set-up using the texture analyzer (TA) CT3-1000 (Brookfield, MA, USA). (A) Commercially available oral thin films: i) Vit B12 ii) Ziminta iii) Listerine Red and iv) Listerine Green (mint). (B) Substrates prepared in the lab using: i) PEG 1450 ii) Cetostearyl alcohol iii) Bees wax shown petri-dish (C) Represents TA probe, with double-sided adhesive tape, and Listerine red oral thin film assessed using Cetostearyl Alcohol.
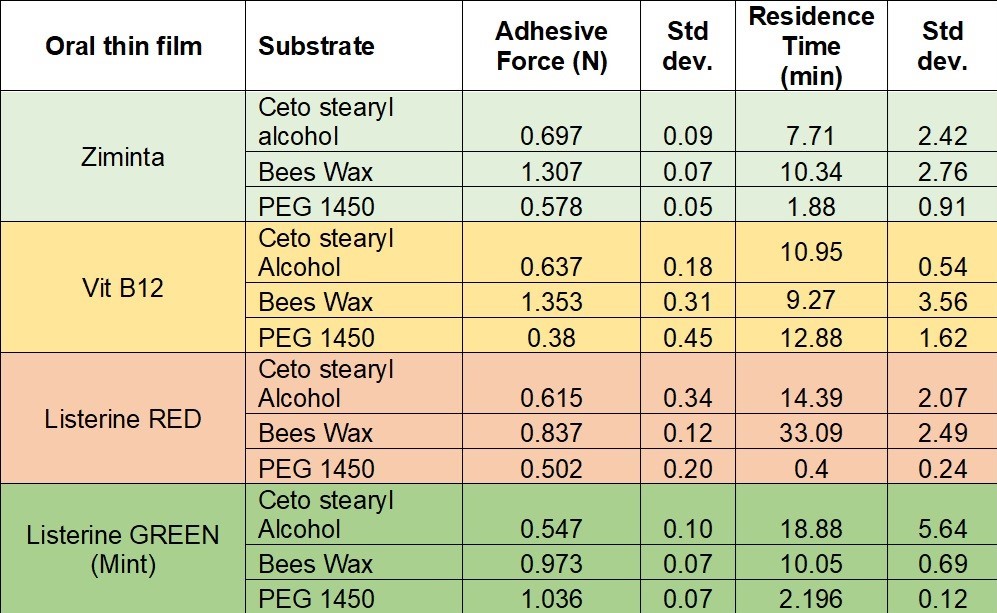 Table 1 showing the results obtained for mucoadhesion and residence time for oral thin films: i) Vit B12 ii) Ziminta iii) Listerine Red and iv) Listerine Green (mint).
Table 1 showing the results obtained for mucoadhesion and residence time for oral thin films: i) Vit B12 ii) Ziminta iii) Listerine Red and iv) Listerine Green (mint).
 Figure 2. The mucoadhesive and residence time test set-up using beaker immersion method. (A) Glass slabs coated with three substrates: i) Beeswax ii) PEG 1450 & iii) Cetostearyl alcohol. (B) Glass slab coated with Beeswax, conditioned with 200 µl of artificial saliva with Listerine red oral thin film pressed on the glass slab immersed into glass beaker of PBS 6.8 at 37 ± 1 °C.
Figure 2. The mucoadhesive and residence time test set-up using beaker immersion method. (A) Glass slabs coated with three substrates: i) Beeswax ii) PEG 1450 & iii) Cetostearyl alcohol. (B) Glass slab coated with Beeswax, conditioned with 200 µl of artificial saliva with Listerine red oral thin film pressed on the glass slab immersed into glass beaker of PBS 6.8 at 37 ± 1 °C.
Methods: Texture Analyzer Preparation: A texture analyzer CT3-1000 (Brookfield, MA, USA) was employed to determine AF. Parameters included a target force of 0.96 N, a hold time of 15 seconds, and a speed of 0.1 mm (about 0 in)/s, with 200 microliters (µl) of artificial saliva [10]. Oral thin film was attached to the probe with double-sided tape. Instrument force, distance, and speed were calibrated. The wax substrate was placed under the probe and 200 µl artificial saliva was used for conditioning purposes. The adhesive behavior (adhesive force) of four OTF formulations against various substrates was investigated with the use of the texture analyzer [10]. Glass slabs were coated with substrate and conditioned with 200 µl of artificial saliva. The OTF was placed on a glass slab. The prepared glass slab was then immersed vertically into a glass beaker containing 200 microliters of phosphate buffer (PBS) pH 6.8 at 37 °C ± 1 °C, followed by stirring at 60 rpm to simulate buccal conditions. Subsequently, the time required for the film to detach completely from the coated glass substrate was observed and recorded as the mucoadhesive retention time (RT). The experiment was conducted in triplicate, and the RT was determined based on the time taken for the film to fully detach from the substrate. [7, 11, 12, 13 & 14].
Results: The adhesive force (AF) was evaluated using three different substrates. PEG 1450 exhibited the lowest AF at 0.38 N for B12 and the highest at 1.036 N for Listerine Green. The adhesive force for Ziminta, B12, and Listerine Red in Cetostearyl alcohol was almost the same at 0.6 N, whereas for Listerine Green, it was slightly lower at 0.547 N. Beeswax yielded similar results for Ziminta and B12 at 1.3 N, with the lowest being Listerine Red at 0.8 N. Overall, Beeswax showed a higher mean AF compared to the other substrates. The residence time (RT) was evaluated using three different substrates. PEG 1450 showed the lowest RT of 0.4 minutes with Listerine Red and the highest of 12.88 minutes for B12. Cetostearyl alcohol ranged from 7.71 minutes (Ziminta) to 18.88 minutes (Listerine Green), indicating a moderate to high affinity for the films. However, residence times varied significantly, with Listerine Red showing the highest value of 33.09 minutes and Ziminta the lowest at 10.34 minutes, suggesting a high variability in film retention. Overall, the mean RT was highest for Beeswax and lowest for PEG 1450 when comparing the three substrates. The Pearson correlation coefficient was calculated and was found to be of approximately 0.888 indicates a strong positive correlation between AF and RT for the Ziminta oral film. For vitamin B12, Listerine Red, and Listerine Green, the results were found to be -0.236, -0.173, and -0.972 respectively. These results for the latter three oral thin films indicate that there is not a strong relationship between AF and RT for these specific films. Additionally, higher adhesive forces are strongly associated with shorter residence times for this film.
Conclusion: Beeswax exhibited the highest mean RT and AF, indicating strong retention on hydrophobic surfaces. Cetostearyl alcohol showed moderate residence times, suggesting its potential as an effective substrate. In contrast, PEG 1450 displayed the lowest RT and AF due to its high hydrophilicity. These findings underscore the importance of substrate selection in the development of buccal drug delivery systems. Hydrophobic substrates like Beeswax tend to enhance mucoadhesion, whereas hydrophilic substrates like PEG 1450 may reduce adhesive performance. Our in vitro method provides an alternative to biological substrates for assessing OTF properties.
References: 1. Pearce, A. K., & O’Reilly, R. K. (2021). Polymers for biomedical applications: The importance of hydrophobicity in directing biological interactions and application efficacy. Biomacromolecules, 22(11), 4459–4469. https://doi.org/10.1021/acs.biomac.1c00434
2. Hartig, T., Mohamed, A. T., Fattah, N. F., Gülses, A., Tjardts, T., Kangah, E. A., Chan, K. P., Veziroglu, S., Acil, Y., Aktas, O. C., Wiltfang, J., Loutfy, S. A., Strunskus, T., Faupel, F., Amin, A., & Schröder, S. (2023). ICVD polymer thin film bio‐interface‐performance for fibroblasts, cancer‐cells, and viruses connected to their functional groups and in silico studies. Advanced Materials Interfaces, 11(1). https://doi.org/10.1002/admi.202300587
3. Laffleur, F. (2014). Mucoadhesive polymers for buccal drug delivery. Drug Development and Industrial Pharmacy, 40(5), 591–598. https://doi.org/10.3109/03639045.2014.892959
4. Nazila Salamat-Miller, Montakarn Chittchang, Thomas P. Johnston. The use of mucoadhesive polymers in buccal drug delivery. Advanced Drug Delivery Reviews.Volume 57, Issue 11, 2005,Pages 1666-1691,ISSN 0169-409X, https://doi.org/10.1016/j.addr.2005.07.003.
5. Wang XQ, Iqbal J, Rahmat D, Bernkop-Schnurch A. Preactivated thiomers: permeation enhancing properties. Int J Pharm 2012;438:217–24
6. Bassi da Silva, J., Ferreira, S. B. de S., de Freitas, O., & Bruschi, M. L. (2017). A critical review of methodologies for the analysis of mucoadhesive properties of drug delivery systems. Drug Development and Industrial Pharmacy, 43(7), 1053–1070. https://doi.org/10.1080/03639045.2017.1294600
7. Alaei S, Omidian H. Mucoadhesion and Mechanical Assessment of Oral Films. Eur J Pharm Sci. 2021 Apr 1; 159: 105727. doi: 10.1016/j.ejps.2021.105727. Epub 2021 Jan 21. PMID: 33484813.
8. Kumar, A., Naik, P. K., Pradhan, D., Ghosh, G., & Rath, G. (2020). Mucoadhesive formulations: innovations, merits, drawbacks, and future outlook. Pharmaceutical Development and Technology, 25(7), 797–814. https://doi.org/10.1080/10837450.2020.1753771
9. Figueiras, A., Pais, A.A.C.C. & Veiga, F.J.B. A Comprehensive Development Strategy in Buccal Drug Delivery. AAPS PharmSciTech 11, 1703–1712 (2010). https://doi.org/10.1208/s12249-010-9546-1
10. Alaei, S., Omidi, Y., & Omidian, H. (2021). In vitro evaluation of adhesion and mechanical properties of oral thin films. European Journal of Pharmaceutical Sciences, 166, 105965.
11. Abruzzo, A., Nicoletta, F. P., Dalena, F., Cerchiara, T., Luppi, B., & Bigucci, F. (2017). Bilayered buccal films as child-appropriate dosage form for systemic administration of propranolol. International journal of pharmaceutics, 531(1), 257-265.
12. Ammar, H. O., Ghorab, M. M., Mahmoud, A. A., & Shahin, H. I. (2017). Design and in vitro/in vivo evaluation of ultra-thin mucoadhesive buccal film containing fluticasone propionate. Aaps Pharmscitech, 18, 93-103.
13. Fithri, A. N., Wijaya, D. P., & Taher, T. (2020). Optimization of chitosan–tapioca starch composite as polymer in the formulation of gingival mucoadhesive patch film for delivery of gambier (Uncaria gambir Roxb) leaf extract. International journal of biological macromolecules, 144, 289-295.
14. Mohamad, S. A., Sarhan, H. A., Abdelkader, H., & Mansour, H. F. (2017). Vitamin B12–loaded buccoadhesive films as a noninvasive supplement in vitamin B12 deficiency: In vitro evaluation and in vivo comparative study with intramuscular injection. Journal of pharmaceutical sciences, 106(7), 1849-1858.
Acknowledgements: Affiliation: - All authors are associated with the college of pharmacy at Nova Southeastern University in Fort Lauderdale, Florida.
Acknowledgements & Author contributions: - We acknowledge Nova Southeastern University facilities for the implementation of the research. HO, SDC, RLW, EJG and AA designed, analyzed, revised the data, and wrote the manuscript. SDC, RLW, EJG and AA conducted the experiments.
Disclaimer: - The statements, opinions and data contained in this abstract are solely those of the individual author(s) and contributor(s) and not of the AAPS, or the reviewer/editor. AAPS and/or the reviewer/editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Funding: - This research received no external funding.
Disclosure or conflict of interest statements: - The authors declare no conflicts of interest.
 Figure 1. The mucoadhesion test set-up using the texture analyzer (TA) CT3-1000 (Brookfield, MA, USA). (A) Commercially available oral thin films: i) Vit B12 ii) Ziminta iii) Listerine Red and iv) Listerine Green (mint). (B) Substrates prepared in the lab using: i) PEG 1450 ii) Cetostearyl alcohol iii) Bees wax shown petri-dish (C) Represents TA probe, with double-sided adhesive tape, and Listerine red oral thin film assessed using Cetostearyl Alcohol.
Figure 1. The mucoadhesion test set-up using the texture analyzer (TA) CT3-1000 (Brookfield, MA, USA). (A) Commercially available oral thin films: i) Vit B12 ii) Ziminta iii) Listerine Red and iv) Listerine Green (mint). (B) Substrates prepared in the lab using: i) PEG 1450 ii) Cetostearyl alcohol iii) Bees wax shown petri-dish (C) Represents TA probe, with double-sided adhesive tape, and Listerine red oral thin film assessed using Cetostearyl Alcohol. Table 1 showing the results obtained for mucoadhesion and residence time for oral thin films: i) Vit B12 ii) Ziminta iii) Listerine Red and iv) Listerine Green (mint).
Table 1 showing the results obtained for mucoadhesion and residence time for oral thin films: i) Vit B12 ii) Ziminta iii) Listerine Red and iv) Listerine Green (mint).  Figure 2. The mucoadhesive and residence time test set-up using beaker immersion method. (A) Glass slabs coated with three substrates: i) Beeswax ii) PEG 1450 & iii) Cetostearyl alcohol. (B) Glass slab coated with Beeswax, conditioned with 200 µl of artificial saliva with Listerine red oral thin film pressed on the glass slab immersed into glass beaker of PBS 6.8 at 37 ± 1 °C.
Figure 2. The mucoadhesive and residence time test set-up using beaker immersion method. (A) Glass slabs coated with three substrates: i) Beeswax ii) PEG 1450 & iii) Cetostearyl alcohol. (B) Glass slab coated with Beeswax, conditioned with 200 µl of artificial saliva with Listerine red oral thin film pressed on the glass slab immersed into glass beaker of PBS 6.8 at 37 ± 1 °C.