Formulation and Delivery - Chemical
(M1330-09-51) Synthesis and Characterization of Nanostructured Lipid Carriers (NLC) with Bile Salts: Sodium Deoxycholate (SDC), Sodium Deoxyglycholate (SDGC) and Sodium Deoxytaurocholate (SDTC)
Monday, October 21, 2024
1:30 PM - 2:30 PM MT
- JM
Javier O. Morales, Ph.D.
Pharmaceutical Sciences PhD
Universidad de Chile
Santiago, Region Metropolitana, Chile 
Gabriela N. Otavalo, BQF (she/her/hers)
Biochemist and pharmacist
Universidad de Chile
Santiago, Region Metropolitana, Chile- JM
Javier O. Morales, Ph.D.
Pharmaceutical Sciences PhD
Universidad de Chile
Santiago, Region Metropolitana, Chile
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Bile salts (BS) are known as edge activators (EA) and permeation enhancers (PE) due to their ability to increase membrane flexibility and deformability. This, in turn, enhances the passage of drugs across the cytoplasmic membrane, thereby improving bioavailability. This study represents the first analysis of BS incorporation with three EA or PE into NLC. The objective of this study was to examine the impact of BS, specifically SDC, SDGC and SDTC on the physicochemical characteristics of NLC, including hydrodynamic diameter (HD), polydispersity index (PdI), Zeta potential (ZP), and stability. This study represents a preliminary analysis of the NLC-BS, which is anticipated to be utilized for buccal permeation.
Methods: A modified method combining low-energy hot emulsification with an injection technique was employed to synthesize modified NLCs with different concentrations of BS. To determine the effect of BS incorporation, the physicochemical characteristics were evaluated, including HD and PdI determined by Dynamic Light Scattering (DLS) and ZP by Laser Doppler Electrophoresis. Furthermore, a stability study was conducted on all formulations, with the same physicochemical characteristics described above being measured up to four weeks after storage at 4 °C. Four different concentrations of BS were tested for SDC (2, 6, 10 and 15 mM), for SDGC and SDTC (2, 6 and 10 mM), which yielded a total of 10 NLC syntheses. Furthermore, empty NLC were synthesized.
Results: The data presented in Figure 2 demonstrate a statistically significant difference in the physicochemical parameters when concentrations of BS are added to the NLC formulations. The NLC exhibited a DH of 137.1 ± 2 nm, a PdI of 0.098 ± 0.026, and a ZP of -11.3 ± 1.6 mV. As illustrated in Fig. 2A, the NLC-SDC exhibited an elevation in HD upon the escalation of the SDC concentration from 126.3 ± 2.7 to 163.8 ± 0.7 nm. This represents a 29.6% increase in HD when the SDC unconjugated BS concentration was raised from 0 to 15 mM. Conversely, a reduction in HD is observed with an increase in the concentration of conjugated BS. Consequently, a reduction in the HD of NLC-SDGC from 176.7 ± 1.4 to 129.3 ± 0.5 nm was observed, representing a 29.6% reduction in HD. Similarly, a reduction in the HD of NLC-SDTC from 186.6 ± 2.1 to 127.3 ± 0.2 nm was observed, representing a 31.8% reduction in HD. This suggests that the incorporation of conjugated BS could potentially reduce the interfacial tension between the oil and aqueous phases of the NLC. Regarding the PdI, it is observed that the NLCs that have a higher concentration of BS have a lower PdI than those that incorporate a lower BS concentration. Fig. 2A illustrates a shift from 0.122 ± 0.017 to 0.070 ± 0.031 for NLC-SDC; 0.088 ± 0.028 to 0.062 ± 0.012 for NLC-SDGC; and 0.238 ± 0.011 to 0.102 ± 0.062 in NLC-SDTC. The PdI demonstrated a downward trend as the concentration of BS increased, with a decrease of approximately 30-40%. Moreover, the PdI demonstrated that all formulations were presented as monodisperse systems. This can be attributed to the stabilizing function of the added BS. About the ZP, a notable increase in the absolute value is observed as the BS concentration increases from 0 to 10 mM. The ZP values become more negative as the BS concentration increases (Fig. 2B). This is evidenced by a change from -15.7 ± 0.7 to -36.6 ± 0.6 for NLC-SDC; -15.7 ± 0.4 to -38.2 ± 1.0 for NLC-SDGC; and -22.4 ± 0.7 to -28.8 ± 1.1 mV for NLC-SDTC due BS are molecules that exhibit the same behavior as anionic surfactants. Consequently, they provide a negative charge in solution, associated with higher electrostatic repulsion and a reduction in particle aggregation. Finally, the stability of the pharmaceutical formulations (Fig. 3 C, D and E) was evaluated over one month at a storage temperature of 4 °C. The results indicated that the three parameters exhibited minimal variation over time. The storage of NLC-BS at a temperature of 4 °C does not significantly affect its physicochemical characteristics. Indeed, minimal variation is observed, contributing to the high stability of the NLC-BS.
Conclusion: The incorporation of BS into NLC generally resulted in a reduction in HD and PdI and an increase in ZP. Furthermore, the stability study demonstrated that NLC-BS are stable for at least four weeks when stored at a temperature of 4 °C. These findings offer insights into the behavior of physicochemical parameters when BS are incorporated into NLC formulations. A range of SB concentrations above the CMC (minimum micellar concentration) was analyzed, with a particular focus on their potential role as edge activators or permeation enhancers. This could be exploited to overcome deficiencies in permeation through cell membranes.
References: -Emzhik, M., et al. (2024). Bile salt-enriched vs. non-enriched nanoparticles. Pharmaceutical Development and Technology. PMID: 38369965.
-Kabir-ud-Din, et al. (2015). Tenside Surfactants Detergents, 52(4), 271–279.
-Ortiz, A. C., et al. (2021). Pharmaceutics. ISSN 1999-4923.
Acknowledgements: Fondecyt N ° 1231154, FONDAP Project 15130011 and Doctoral Scholarship from the University of Chile 2024.
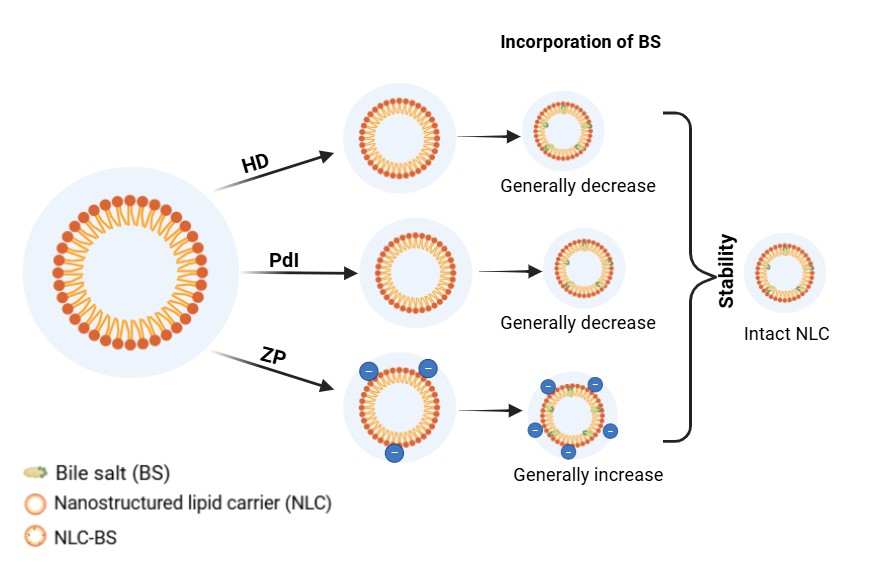 Figure 1. NLC modified with bile salts and analysis of their physicochemical characteristics.
Figure 1. NLC modified with bile salts and analysis of their physicochemical characteristics.
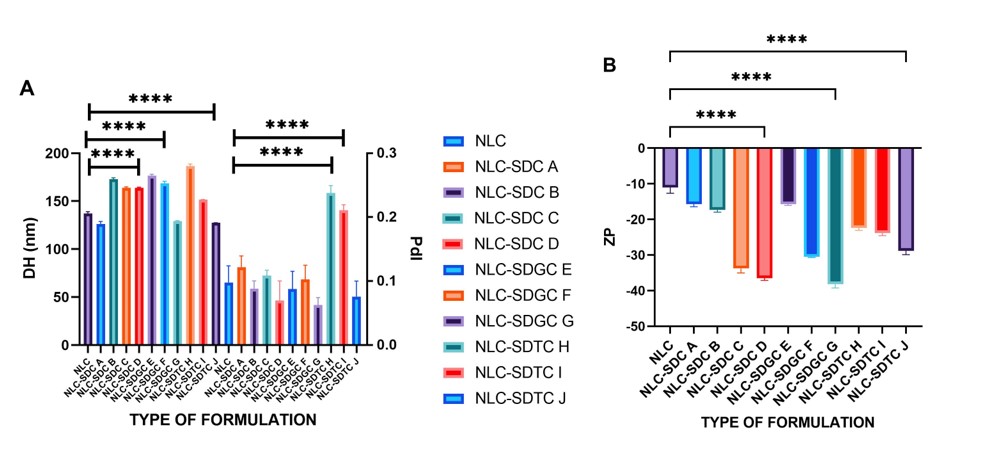 Figure 2. The incorporation of bile salts into NLC formulations has been observed to influence the HD, PdI and ZP of the NLC-BS formulations. A . Hydrodynamic diameter and PdI of NLC-BS formulations with different concentrations of SDC (NLC-SDC A = 2 mM; NLC-SDC B = 6 mM NLC-SDC C = 10 Mm y NLC-SDC D = 15 mM); SDGC (NLC-SDGC E = 2 mM; NLC-SGDC F = 6 mM NLC-SGDC G = 10 Mm) and SDTC (NLC-SDTC H = 2 mM; NLC-STDC I= 6 mM NLC-STDC J = 10 mM). The decrease in HD and PdI is observed with increasing concentration of SDGC and SDTC. Likewise, the increase in HD is also observed with increasing SDC concentration. B. Zeta potential of NLC-BS formulations with the same concentrations indicated above. The increase in ZP is observed with increasing BS concentration. p < .001.
Figure 2. The incorporation of bile salts into NLC formulations has been observed to influence the HD, PdI and ZP of the NLC-BS formulations. A . Hydrodynamic diameter and PdI of NLC-BS formulations with different concentrations of SDC (NLC-SDC A = 2 mM; NLC-SDC B = 6 mM NLC-SDC C = 10 Mm y NLC-SDC D = 15 mM); SDGC (NLC-SDGC E = 2 mM; NLC-SGDC F = 6 mM NLC-SGDC G = 10 Mm) and SDTC (NLC-SDTC H = 2 mM; NLC-STDC I= 6 mM NLC-STDC J = 10 mM). The decrease in HD and PdI is observed with increasing concentration of SDGC and SDTC. Likewise, the increase in HD is also observed with increasing SDC concentration. B. Zeta potential of NLC-BS formulations with the same concentrations indicated above. The increase in ZP is observed with increasing BS concentration. p < .001.
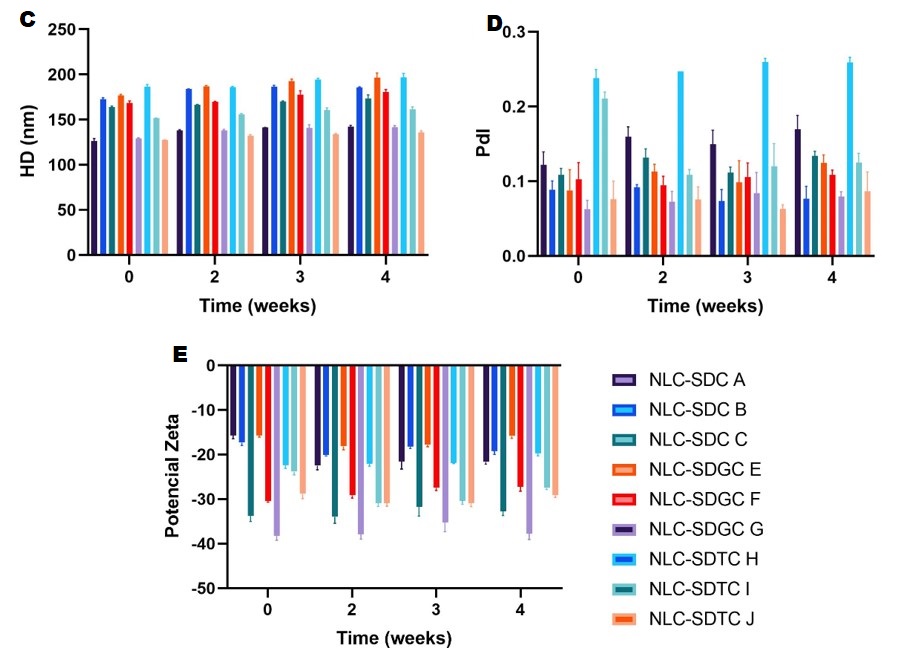 Figure 3. C, D and E. Storage stability tests of the NLC formulations with different concentrations of BS. A.HD. B. PdI. C. Zeta Potential. ° T= 4 °C. The three physicochemical parameters showed minimal variation until the fourth week of storage at 4 °C. The graph depicts the effects of modifying NLCs with SDC, SDGC, and STDC at concentrations of 2, 6, and 10 mM.
Figure 3. C, D and E. Storage stability tests of the NLC formulations with different concentrations of BS. A.HD. B. PdI. C. Zeta Potential. ° T= 4 °C. The three physicochemical parameters showed minimal variation until the fourth week of storage at 4 °C. The graph depicts the effects of modifying NLCs with SDC, SDGC, and STDC at concentrations of 2, 6, and 10 mM.
Methods: A modified method combining low-energy hot emulsification with an injection technique was employed to synthesize modified NLCs with different concentrations of BS. To determine the effect of BS incorporation, the physicochemical characteristics were evaluated, including HD and PdI determined by Dynamic Light Scattering (DLS) and ZP by Laser Doppler Electrophoresis. Furthermore, a stability study was conducted on all formulations, with the same physicochemical characteristics described above being measured up to four weeks after storage at 4 °C. Four different concentrations of BS were tested for SDC (2, 6, 10 and 15 mM), for SDGC and SDTC (2, 6 and 10 mM), which yielded a total of 10 NLC syntheses. Furthermore, empty NLC were synthesized.
Results: The data presented in Figure 2 demonstrate a statistically significant difference in the physicochemical parameters when concentrations of BS are added to the NLC formulations. The NLC exhibited a DH of 137.1 ± 2 nm, a PdI of 0.098 ± 0.026, and a ZP of -11.3 ± 1.6 mV. As illustrated in Fig. 2A, the NLC-SDC exhibited an elevation in HD upon the escalation of the SDC concentration from 126.3 ± 2.7 to 163.8 ± 0.7 nm. This represents a 29.6% increase in HD when the SDC unconjugated BS concentration was raised from 0 to 15 mM. Conversely, a reduction in HD is observed with an increase in the concentration of conjugated BS. Consequently, a reduction in the HD of NLC-SDGC from 176.7 ± 1.4 to 129.3 ± 0.5 nm was observed, representing a 29.6% reduction in HD. Similarly, a reduction in the HD of NLC-SDTC from 186.6 ± 2.1 to 127.3 ± 0.2 nm was observed, representing a 31.8% reduction in HD. This suggests that the incorporation of conjugated BS could potentially reduce the interfacial tension between the oil and aqueous phases of the NLC. Regarding the PdI, it is observed that the NLCs that have a higher concentration of BS have a lower PdI than those that incorporate a lower BS concentration. Fig. 2A illustrates a shift from 0.122 ± 0.017 to 0.070 ± 0.031 for NLC-SDC; 0.088 ± 0.028 to 0.062 ± 0.012 for NLC-SDGC; and 0.238 ± 0.011 to 0.102 ± 0.062 in NLC-SDTC. The PdI demonstrated a downward trend as the concentration of BS increased, with a decrease of approximately 30-40%. Moreover, the PdI demonstrated that all formulations were presented as monodisperse systems. This can be attributed to the stabilizing function of the added BS. About the ZP, a notable increase in the absolute value is observed as the BS concentration increases from 0 to 10 mM. The ZP values become more negative as the BS concentration increases (Fig. 2B). This is evidenced by a change from -15.7 ± 0.7 to -36.6 ± 0.6 for NLC-SDC; -15.7 ± 0.4 to -38.2 ± 1.0 for NLC-SDGC; and -22.4 ± 0.7 to -28.8 ± 1.1 mV for NLC-SDTC due BS are molecules that exhibit the same behavior as anionic surfactants. Consequently, they provide a negative charge in solution, associated with higher electrostatic repulsion and a reduction in particle aggregation. Finally, the stability of the pharmaceutical formulations (Fig. 3 C, D and E) was evaluated over one month at a storage temperature of 4 °C. The results indicated that the three parameters exhibited minimal variation over time. The storage of NLC-BS at a temperature of 4 °C does not significantly affect its physicochemical characteristics. Indeed, minimal variation is observed, contributing to the high stability of the NLC-BS.
Conclusion: The incorporation of BS into NLC generally resulted in a reduction in HD and PdI and an increase in ZP. Furthermore, the stability study demonstrated that NLC-BS are stable for at least four weeks when stored at a temperature of 4 °C. These findings offer insights into the behavior of physicochemical parameters when BS are incorporated into NLC formulations. A range of SB concentrations above the CMC (minimum micellar concentration) was analyzed, with a particular focus on their potential role as edge activators or permeation enhancers. This could be exploited to overcome deficiencies in permeation through cell membranes.
References: -Emzhik, M., et al. (2024). Bile salt-enriched vs. non-enriched nanoparticles. Pharmaceutical Development and Technology. PMID: 38369965.
-Kabir-ud-Din, et al. (2015). Tenside Surfactants Detergents, 52(4), 271–279.
-Ortiz, A. C., et al. (2021). Pharmaceutics. ISSN 1999-4923.
Acknowledgements: Fondecyt N ° 1231154, FONDAP Project 15130011 and Doctoral Scholarship from the University of Chile 2024.
 Figure 1. NLC modified with bile salts and analysis of their physicochemical characteristics.
Figure 1. NLC modified with bile salts and analysis of their physicochemical characteristics. Figure 2. The incorporation of bile salts into NLC formulations has been observed to influence the HD, PdI and ZP of the NLC-BS formulations. A . Hydrodynamic diameter and PdI of NLC-BS formulations with different concentrations of SDC (NLC-SDC A = 2 mM; NLC-SDC B = 6 mM NLC-SDC C = 10 Mm y NLC-SDC D = 15 mM); SDGC (NLC-SDGC E = 2 mM; NLC-SGDC F = 6 mM NLC-SGDC G = 10 Mm) and SDTC (NLC-SDTC H = 2 mM; NLC-STDC I= 6 mM NLC-STDC J = 10 mM). The decrease in HD and PdI is observed with increasing concentration of SDGC and SDTC. Likewise, the increase in HD is also observed with increasing SDC concentration. B. Zeta potential of NLC-BS formulations with the same concentrations indicated above. The increase in ZP is observed with increasing BS concentration. p < .001.
Figure 2. The incorporation of bile salts into NLC formulations has been observed to influence the HD, PdI and ZP of the NLC-BS formulations. A . Hydrodynamic diameter and PdI of NLC-BS formulations with different concentrations of SDC (NLC-SDC A = 2 mM; NLC-SDC B = 6 mM NLC-SDC C = 10 Mm y NLC-SDC D = 15 mM); SDGC (NLC-SDGC E = 2 mM; NLC-SGDC F = 6 mM NLC-SGDC G = 10 Mm) and SDTC (NLC-SDTC H = 2 mM; NLC-STDC I= 6 mM NLC-STDC J = 10 mM). The decrease in HD and PdI is observed with increasing concentration of SDGC and SDTC. Likewise, the increase in HD is also observed with increasing SDC concentration. B. Zeta potential of NLC-BS formulations with the same concentrations indicated above. The increase in ZP is observed with increasing BS concentration. p < .001. Figure 3. C, D and E. Storage stability tests of the NLC formulations with different concentrations of BS. A.HD. B. PdI. C. Zeta Potential. ° T= 4 °C. The three physicochemical parameters showed minimal variation until the fourth week of storage at 4 °C. The graph depicts the effects of modifying NLCs with SDC, SDGC, and STDC at concentrations of 2, 6, and 10 mM.
Figure 3. C, D and E. Storage stability tests of the NLC formulations with different concentrations of BS. A.HD. B. PdI. C. Zeta Potential. ° T= 4 °C. The three physicochemical parameters showed minimal variation until the fourth week of storage at 4 °C. The graph depicts the effects of modifying NLCs with SDC, SDGC, and STDC at concentrations of 2, 6, and 10 mM.